Flash point is the lowest temperature at which a liquid produces enough vapor to ignite momentarily when exposed to an open flame, while ignition temperature is the minimum temperature required to sustain combustion without an external ignition source. Understanding these differences helps you handle flammable materials safely and prevent accidental fires.
Table of Comparison
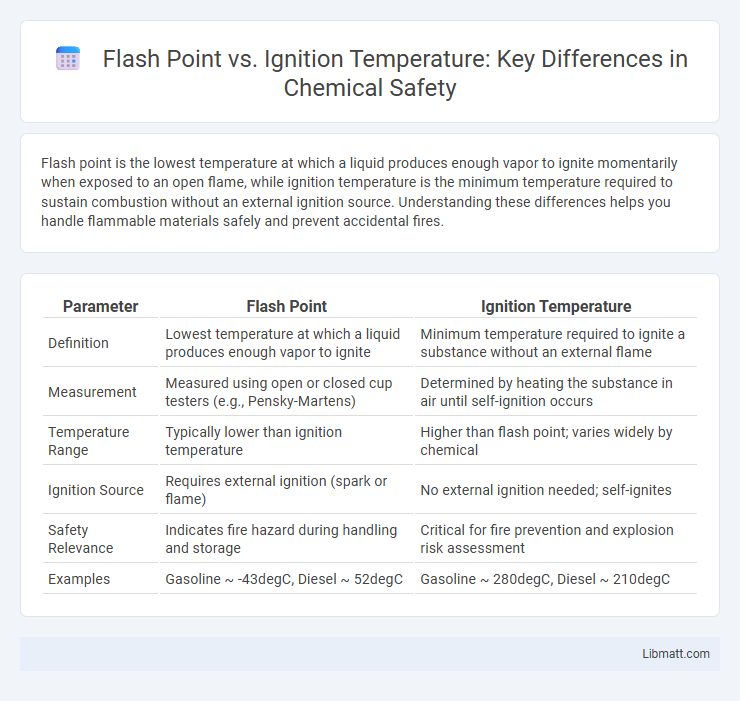
| Parameter | Flash Point | Ignition Temperature |
|---|---|---|
| Definition | Lowest temperature at which a liquid produces enough vapor to ignite | Minimum temperature required to ignite a substance without an external flame |
| Measurement | Measured using open or closed cup testers (e.g., Pensky-Martens) | Determined by heating the substance in air until self-ignition occurs |
| Temperature Range | Typically lower than ignition temperature | Higher than flash point; varies widely by chemical |
| Ignition Source | Requires external ignition (spark or flame) | No external ignition needed; self-ignites |
| Safety Relevance | Indicates fire hazard during handling and storage | Critical for fire prevention and explosion risk assessment |
| Examples | Gasoline ~ -43degC, Diesel ~ 52degC | Gasoline ~ 280degC, Diesel ~ 210degC |
Understanding Flash Point and Ignition Temperature
Flash point indicates the lowest temperature at which a liquid produces enough vapor to form a combustible mixture with air near its surface. Ignition temperature, or autoignition temperature, is the minimum temperature required to ignite the vapor without an external flame or spark. Understanding these critical safety parameters helps you handle flammable substances safely and prevent accidental fires.
Definitions: Flash Point Explained
Flash point refers to the lowest temperature at which a liquid produces enough vapor to ignite momentarily when exposed to an ignition source, indicating its flammability risk. Ignition temperature, or autoignition temperature, is the minimum temperature at which a substance ignites spontaneously without an external flame or spark. Understanding the flash point helps you assess fire hazards during storage and handling of combustible liquids.
Definitions: Ignition Temperature Explained
Ignition temperature, also known as autoignition temperature, is the minimum temperature at which a substance spontaneously ignites without an external flame or spark. This temperature is critical for understanding fire hazards because it indicates when materials will combust independently. Your safety measures depend on knowing the ignition temperature to prevent accidental fires during storage or handling of flammable substances.
Key Differences Between Flash Point and Ignition Temperature
Flash point refers to the lowest temperature at which a liquid produces enough vapor to ignite momentarily in the presence of an ignition source, while ignition temperature is the minimum temperature required to ignite a substance without an external flame or spark. Your understanding of these properties is crucial for safety, as flash point determines flammability during handling and storage, whereas ignition temperature indicates the risk of spontaneous combustion. The key difference lies in that flash point involves vapor ignition with an external source, whereas ignition temperature involves the substance igniting purely from heat.
Importance in Fire Safety and Prevention
Understanding the flash point and ignition temperature is crucial for fire safety and prevention because the flash point indicates the lowest temperature at which a liquid emits enough vapor to ignite in air, allowing early detection of potential fire hazards. The ignition temperature, being higher, represents the minimum heat required to spontaneously ignite a substance without an external flame, helping to establish safe handling and storage conditions. Accurate knowledge of these parameters enables better risk assessment, the design of safer industrial processes, and effective emergency response strategies.
Factors Influencing Flash Point and Ignition Temperature
Flash point and ignition temperature are influenced by factors such as chemical composition, vapor pressure, and environmental conditions like ambient temperature and pressure. Higher volatility and lower molecular weight typically reduce the flash point, while ignition temperature depends on the activation energy required for combustion. Contaminants, oxygen concentration, and surface area exposure also affect these critical thermal properties.
Testing Methods for Flash Point
Flash point testing methods primarily include the closed cup and open cup techniques, with the Pensky-Martens and Tag Closed Cup testers being among the most accurate for determining the lowest temperature at which a liquid emits enough vapor to ignite. Closed cup testers trap vapors, providing safer, more consistent results closely resembling real-world conditions, while open cup methods expose the sample to air, often resulting in higher flash point values. Understanding these testing methods helps you accurately assess the ignition risk and safe handling temperatures of flammable liquids in industrial and laboratory settings.
Testing Methods for Ignition Temperature
Ignition temperature testing methods typically involve gradually heating a substance in a controlled environment until it spontaneously ignites without an external flame or spark. Common techniques include the ASTM E659 cup method and the CMR (Closed Manifold Reactor) method, which provide precise data for safety and handling regulations. Understanding these testing protocols helps you accurately assess fire hazards and prevent accidental ignition in industrial settings.
Real-World Applications and Industry Standards
The flash point and ignition temperature are critical parameters in fire safety management across industries such as petrochemical, manufacturing, and transportation. Flash point determines the minimum temperature at which a liquid emits enough vapor to ignite, guiding storage and handling procedures to prevent accidental fires. Ignition temperature, the minimum temperature required to sustain combustion without an external flame, informs design standards for equipment and sets safety thresholds in regulations like OSHA and NFPA.
Flash Point vs Ignition Temperature: Summary Table
The flash point is the lowest temperature at which a liquid produces enough vapor to ignite momentarily in air, whereas the ignition temperature is the minimum temperature required for a substance to sustain combustion without an external flame. Flash point values for common fuels like gasoline range around -43degC, while ignition temperatures for hydrocarbons often exceed 200degC. A summary table highlights key differences: flash point indicates flammability risk during handling, ignition temperature determines the threshold for spontaneous combustion.
Flash point vs ignition temperature Infographic
 libmatt.com
libmatt.com