Homogeneous catalysts operate in the same phase as the reactants, providing uniform mixing and typically faster reaction rates, while heterogeneous catalysts function in a different phase, often solid catalysts interacting with liquid or gas reactants, facilitating easier separation and reuse. Your catalyst choice depends on factors such as reaction conditions, desired selectivity, and ease of catalyst recovery.
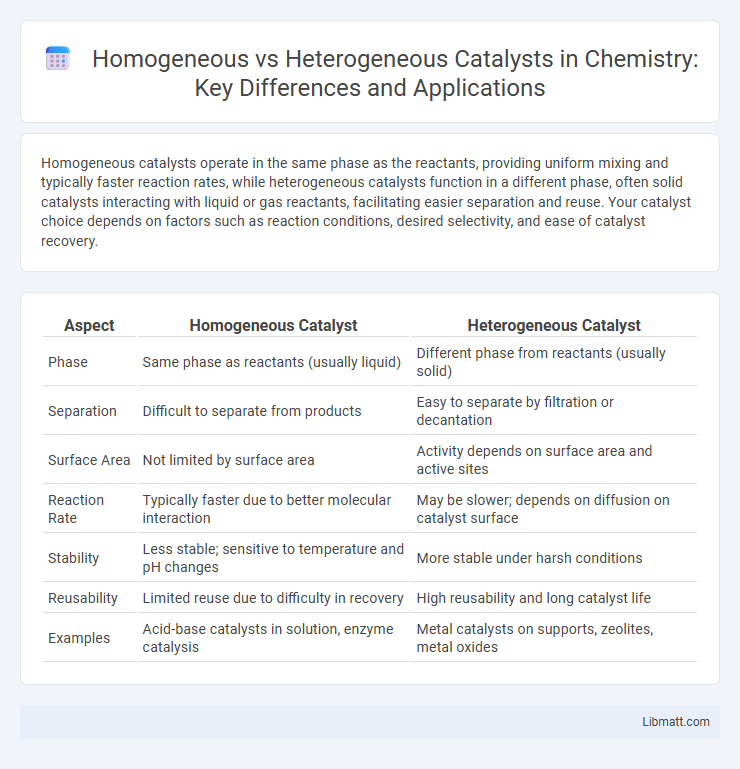
Table of Comparison
| Aspect | Homogeneous Catalyst | Heterogeneous Catalyst |
|---|---|---|
| Phase | Same phase as reactants (usually liquid) | Different phase from reactants (usually solid) |
| Separation | Difficult to separate from products | Easy to separate by filtration or decantation |
| Surface Area | Not limited by surface area | Activity depends on surface area and active sites |
| Reaction Rate | Typically faster due to better molecular interaction | May be slower; depends on diffusion on catalyst surface |
| Stability | Less stable; sensitive to temperature and pH changes | More stable under harsh conditions |
| Reusability | Limited reuse due to difficulty in recovery | High reusability and long catalyst life |
| Examples | Acid-base catalysts in solution, enzyme catalysis | Metal catalysts on supports, zeolites, metal oxides |
Introduction to Catalysts
Catalysts accelerate chemical reactions without being consumed, categorized mainly into homogeneous and heterogeneous types based on their phase relative to the reactants. Homogeneous catalysts exist in the same phase as the reactants, typically liquid, enabling uniform interaction at the molecular level, while heterogeneous catalysts are in a different phase, often solid, providing surface sites for reactant molecules to adsorb and react. Understanding these distinctions helps optimize Your reaction conditions and catalytic efficiency for industrial or laboratory applications.
Definition of Homogeneous Catalysts
Homogeneous catalysts are substances that exist in the same phase as the reactants, typically both being in the liquid phase, allowing for molecular-level interactions that enhance reaction rates. These catalysts often consist of metal complexes dissolved in the reaction mixture, facilitating precise control over reaction pathways and selectivity. Their solubility enables uniform distribution throughout the reaction medium, promoting efficient catalytic activity in processes such as hydroformylation and hydrogenation.
Definition of Heterogeneous Catalysts
Heterogeneous catalysts are substances that accelerate chemical reactions while existing in a different phase from the reactants, typically solid catalysts interacting with liquid or gas reactants. These catalysts provide active sites on their surfaces where reactant molecules adsorb, react, and desorb, making surface area and pore structure critical for catalytic efficiency. Common examples include metal catalysts such as platinum or nickel used in industrial processes like hydrogenation and catalytic converters.
Key Differences Between Homogeneous and Heterogeneous Catalysts
Homogeneous catalysts are in the same phase as the reactants, typically liquid, facilitating molecular-level interactions that enhance reaction rates and selectivity. Heterogeneous catalysts exist in a different phase, often solid catalysts interacting with gaseous or liquid reactants at active surface sites, enabling easier separation and reuse. Your choice between these catalysts depends on factors like reaction conditions, catalyst recovery, and product purity, with homogeneous systems offering precise control and heterogeneous ones providing mechanical stability and recyclability.
Mechanisms of Catalytic Action
Homogeneous catalysts operate via molecular interaction with reactants in a single phase, facilitating mechanisms such as ligand exchange, oxidative addition, and reductive elimination that enable precise control over reaction pathways. Heterogeneous catalysts function through surface adsorption and activation of reactants on solid interfaces, promoting mechanisms like adsorption-desorption cycles, surface diffusion, and active site regeneration. The distinction in catalytic action mechanisms fundamentally influences factors such as selectivity, reaction rates, and catalyst recyclability in chemical processes.
Advantages of Homogeneous Catalysts
Homogeneous catalysts offer superior selectivity and reaction rates due to their molecular-level interaction with reactants, enabling precise control over reaction pathways. Their solubility in the reaction medium allows for uniform distribution, leading to enhanced efficiency and easier optimization of reaction conditions. These catalysts also facilitate mechanistic studies and catalyst modification, promoting innovation in catalytic processes.
Advantages of Heterogeneous Catalysts
Heterogeneous catalysts offer significant advantages such as ease of separation from reaction mixtures, allowing for efficient catalyst recovery and reuse. Their solid state provides enhanced stability under harsh reaction conditions, leading to longer catalyst lifetimes. Additionally, heterogeneous catalysts enable continuous processing in industrial applications, improving scalability and operational efficiency.
Challenges and Limitations
Homogeneous catalysts often face challenges in separation and recycling due to their solubility in the reaction medium, leading to difficulties in catalyst recovery and potential contamination of products. Heterogeneous catalysts, while easier to separate and reuse, are limited by lower selectivity and mass transfer issues caused by surface availability and diffusion constraints. Both catalyst types encounter stability problems under extreme reaction conditions, affecting their long-term efficiency and operational lifespan.
Industrial Applications and Case Studies
Homogeneous catalysts, typically used in fine chemical synthesis such as pharmaceutical production, provide high selectivity and ease of modification but pose challenges in separation and recycling. Heterogeneous catalysts dominate large-scale industrial processes like petrochemical refining and ammonia synthesis due to their durability, ease of separation, and reusability. Your choice between these catalysts depends on process scale, desired product purity, and economic factors highlighted in case studies like the Haber-Bosch process for heterogeneous catalysis and hydroformylation reactions for homogeneous catalysis.
Future Trends in Catalyst Development
Future trends in catalyst development emphasize the integration of homogeneous and heterogeneous catalyst properties to enhance selectivity, stability, and recyclability. Advances in nanotechnology and materials science enable the design of hybrid catalysts that combine the molecular precision of homogeneous catalysts with the robustness of heterogeneous catalysts for industrial applications. Research is increasingly focused on sustainable catalysts that operate under mild conditions, reduce waste, and utilize abundant, non-toxic elements to meet environmental and economic demands.
Homogeneous catalyst vs heterogeneous catalyst Infographic
 libmatt.com
libmatt.com