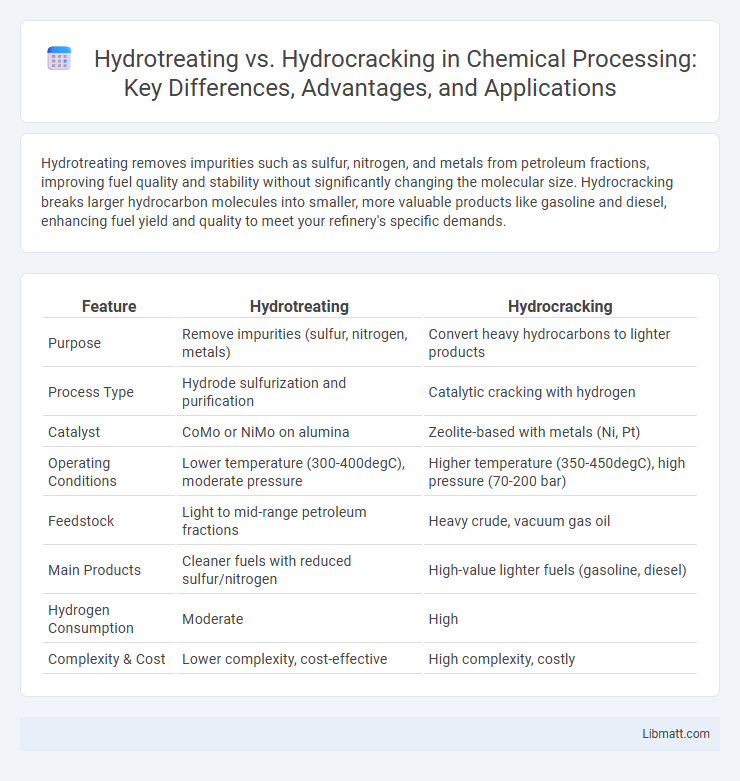
Hydrotreating removes impurities such as sulfur, nitrogen, and metals from petroleum fractions, improving fuel quality and stability without significantly changing the molecular size. Hydrocracking breaks larger hydrocarbon molecules into smaller, more valuable products like gasoline and diesel, enhancing fuel yield and quality to meet your refinery's specific demands.
Table of Comparison
| Feature | Hydrotreating | Hydrocracking |
|---|---|---|
| Purpose | Remove impurities (sulfur, nitrogen, metals) | Convert heavy hydrocarbons to lighter products |
| Process Type | Hydrode sulfurization and purification | Catalytic cracking with hydrogen |
| Catalyst | CoMo or NiMo on alumina | Zeolite-based with metals (Ni, Pt) |
| Operating Conditions | Lower temperature (300-400degC), moderate pressure | Higher temperature (350-450degC), high pressure (70-200 bar) |
| Feedstock | Light to mid-range petroleum fractions | Heavy crude, vacuum gas oil |
| Main Products | Cleaner fuels with reduced sulfur/nitrogen | High-value lighter fuels (gasoline, diesel) |
| Hydrogen Consumption | Moderate | High |
| Complexity & Cost | Lower complexity, cost-effective | High complexity, costly |
Introduction to Hydrotreating and Hydrocracking
Hydrotreating and hydrocracking are essential refining processes that improve the quality and yield of petroleum products by treating hydrocarbons with hydrogen under high pressure and temperature. Hydrotreating primarily removes sulfur, nitrogen, and metal contaminants to produce cleaner fuels, while hydrocracking breaks larger hydrocarbon molecules into smaller, more valuable fractions like gasoline and diesel. Understanding these processes helps you optimize refinery output and meet stringent environmental regulations.
Fundamentals of Hydrotreating
Hydrotreating is a catalytic chemical process that removes sulfur, nitrogen, oxygen, and metals from petroleum fractions through hydrogenation reactions under high pressure and moderate temperature. It primarily uses catalysts such as cobalt-molybdenum or nickel-molybdenum on alumina supports to improve fuel quality and meet environmental standards by reducing contaminants. This process serves as a crucial pretreatment step in refining before more severe treatments like hydrocracking.
Fundamentals of Hydrocracking
Hydrocracking is a catalytic process that breaks down heavy hydrocarbons into lighter, more valuable products like gasoline, diesel, and jet fuel by using hydrogen under high pressure and temperature. The process involves catalytic cracking combined with hydrogenation, which saturates olefins and aromatics to improve product stability and quality. Unlike hydrotreating, which primarily removes impurities such as sulfur and nitrogen, hydrocracking significantly alters the molecular structure to upgrade feedstock into premium fuels.
Key Process Differences
Hydrotreating primarily removes sulfur, nitrogen, and metals from feedstocks through catalytic hydrogenation to improve fuel quality, whereas hydrocracking breaks larger, heavier hydrocarbon molecules into lighter, more valuable products like gasoline and diesel. The hydrotreating process operates under milder conditions focusing on impurity removal and saturation of olefins, while hydrocracking uses higher pressure and temperature to crack heavy molecules and enhance yield of lighter fractions. Your choice between these processes depends on feedstock characteristics and desired product slate, balancing impurity removal with hydrocarbon upgrading.
Feedstock Considerations
Hydrotreating primarily processes feedstocks with lower complexity such as straight-run gas oils or light catalytic cycle oils to remove sulfur, nitrogen, and metals, improving fuel quality. Hydrocracking handles heavier, more complex feedstocks including vacuum gas oils and residual oils by breaking large molecules into lighter, valuable products like diesel and jet fuel. Feedstock selection impacts catalyst choice, operating conditions, and overall product yield in both processes.
Catalyst Types and Functions
Hydrotreating catalysts primarily consist of cobalt-molybdenum (Co-Mo) or nickel-molybdenum (Ni-Mo) supported on alumina, designed to remove sulfur, nitrogen, and metals by hydrogenation and hydrodesulfurization reactions, thereby improving fuel quality and meeting environmental regulations. Hydrocracking catalysts generally combine acidic supports such as zeolites with metals like platinum or palladium to facilitate both hydrogenation and cracking processes, effectively breaking down heavy hydrocarbons into lighter, more valuable products like gasoline and jet fuel. The catalytic function in hydrotreating is mainly focused on impurity removal, whereas hydrocracking catalysts optimize hydrocarbon cracking and saturation simultaneously, enhancing product yield and selectivity.
Product Yield and Quality
Hydrotreating primarily improves product quality by removing sulfur, nitrogen, and metals, resulting in cleaner fuels with higher stability but offers limited changes to product yield. Hydrocracking significantly enhances product yield by breaking larger molecules into valuable lighter fractions like gasoline and diesel, while also improving quality through simultaneous hydrodesulfurization and saturation of aromatics. Your choice between these processes depends on whether higher yield of lighter, premium fuels or upgrading feedstock quality is the operational priority.
Environmental Impact and Emissions
Hydrotreating significantly reduces sulfur, nitrogen, and other contaminants in petroleum products, leading to lower emissions of sulfur oxides (SOx) and nitrogen oxides (NOx) during fuel combustion. Hydrocracking produces cleaner, higher-quality fuels by breaking down heavier hydrocarbons but typically requires more energy, which can increase indirect CO2 emissions if powered by fossil fuels. Your choice between hydrotreating and hydrocracking impacts the environmental footprint of fuel production, with hydrotreating offering targeted pollutant removal and hydrocracking contributing to cleaner end-use fuel combustion.
Industrial Applications and Use Cases
Hydrotreating is primarily used in petroleum refineries to remove sulfur, nitrogen, and other contaminants from crude oil fractions, improving fuel quality and meeting environmental regulations. Hydrocracking is applied to heavier feedstocks such as vacuum gas oils and residual oils, converting them into high-value products like diesel, jet fuel, and naphtha with superior octane and cetane numbers. Both processes are integral in producing cleaner fuels and petrochemical feedstocks, with hydrotreating focusing on purification and hydrocracking on molecular restructuring for enhanced product yield.
Future Trends in Hydrotreating and Hydrocracking
Future trends in hydrotreating emphasize enhanced catalyst development for improved sulfur removal and reduced environmental impact, meeting stricter fuel regulations. Hydrocracking advancements focus on integrated processes combining hydrocracking with renewable feedstocks to produce higher yields of diesel and jet fuels. Both technologies are increasingly adopting digitalization and process intensification to optimize efficiency and reduce operational costs in refining.
Hydrotreating vs hydrocracking Infographic
 libmatt.com
libmatt.com