Ionic liquids are salts in a liquid state at relatively low temperatures, known for their high thermal stability and conductivity, while deep eutectic solvents (DES) are mixtures of hydrogen bond donors and acceptors that form a eutectic with a melting point lower than either component. Your choice depends on the application needs for eco-friendly solvents, as ionic liquids offer tunable properties and DES provide cost-effective, biodegradable alternatives.
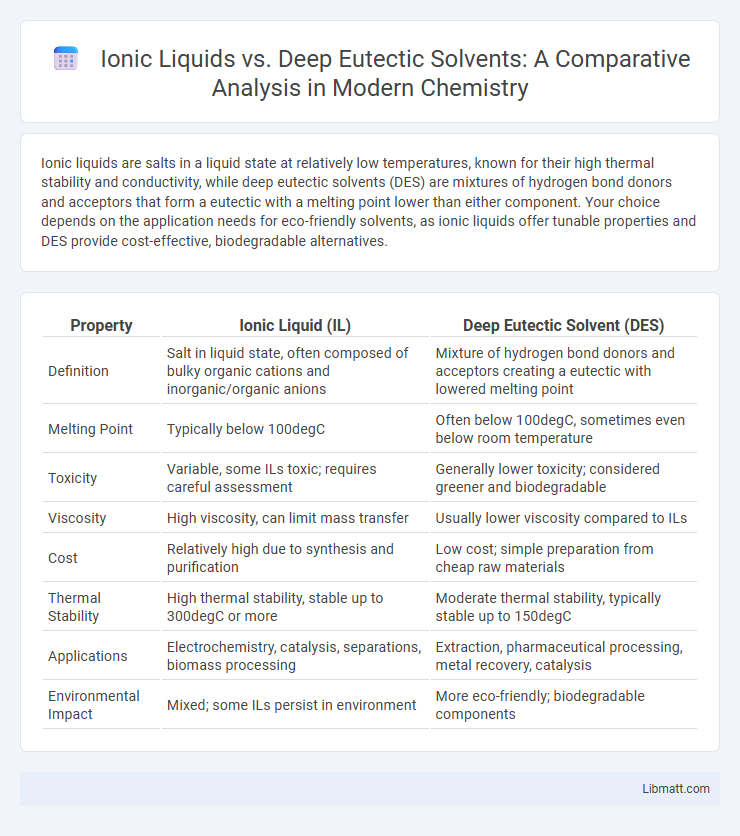
Table of Comparison
| Property | Ionic Liquid (IL) | Deep Eutectic Solvent (DES) |
|---|---|---|
| Definition | Salt in liquid state, often composed of bulky organic cations and inorganic/organic anions | Mixture of hydrogen bond donors and acceptors creating a eutectic with lowered melting point |
| Melting Point | Typically below 100degC | Often below 100degC, sometimes even below room temperature |
| Toxicity | Variable, some ILs toxic; requires careful assessment | Generally lower toxicity; considered greener and biodegradable |
| Viscosity | High viscosity, can limit mass transfer | Usually lower viscosity compared to ILs |
| Cost | Relatively high due to synthesis and purification | Low cost; simple preparation from cheap raw materials |
| Thermal Stability | High thermal stability, stable up to 300degC or more | Moderate thermal stability, typically stable up to 150degC |
| Applications | Electrochemistry, catalysis, separations, biomass processing | Extraction, pharmaceutical processing, metal recovery, catalysis |
| Environmental Impact | Mixed; some ILs persist in environment | More eco-friendly; biodegradable components |
Introduction to Ionic Liquids and Deep Eutectic Solvents
Ionic liquids are salts with melting points below 100degC, known for their low volatility and high thermal stability, making them ideal solvents in green chemistry. Deep eutectic solvents are mixtures of two or more components that form a eutectic with a melting point significantly lower than that of the individual components, offering cost-effective and biodegradable alternatives. Your selection between these solvents depends on factors like toxicity, environmental impact, and application requirements in fields such as catalysis and electrochemistry.
Chemical Structures and Compositions
Ionic liquids consist of discrete ions, typically large organic cations paired with inorganic or organic anions, exhibiting low melting points below 100degC due to their unique ionic interactions. Deep eutectic solvents (DES) arise from hydrogen bond interactions between a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), often involving components like choline chloride mixed with urea or glycerol, resulting in a eutectic mixture with a melting point significantly lower than its individual constituents. Understanding these chemical structures and compositions helps you select the appropriate solvent for applications requiring tailored solvent properties and green chemistry principles.
Synthesis Methods and Scalability
Ionic liquids are typically synthesized via quaternization reactions followed by anion exchange, allowing tunable properties but often involving costly purification steps; deep eutectic solvents (DES) form by simply mixing hydrogen bond donors and acceptors, offering a straightforward, low-cost, and energy-efficient synthesis. In terms of scalability, DES production is more amenable to large-scale industrial processes due to simpler preparation and safer handling, whereas ionic liquid scalability is limited by complex synthesis routes and higher raw material expenses. The sustainable and scalable nature of DES positions them as attractive alternatives to ionic liquids in green chemistry applications.
Physical and Chemical Properties
Ionic liquids exhibit low volatility, high thermal stability, and excellent ionic conductivity due to their ionic nature and strong Coulombic interactions. Deep eutectic solvents (DES) display tunable viscosity and melting points, often lower than their individual components, resulting from hydrogen bonding between their constituents. Your choice between these solvents depends on balancing the need for chemical stability and ionic mobility with desired physical characteristics like viscosity and melting behavior.
Environmental Impact and Biodegradability
Ionic liquids often exhibit low volatility but can pose environmental concerns due to their potential toxicity and persistence in ecosystems, making biodegradability a critical challenge. Deep eutectic solvents (DESs) are generally more environmentally friendly, derived from biodegradable and naturally occurring components that reduce toxicity and improve sustainability. Choosing DESs can enhance your applications by minimizing ecological impact while maintaining effective solvent properties.
Applications in Green Chemistry
Ionic liquids offer exceptional thermal stability and tunable solvation properties, making them ideal for catalysis, carbon capture, and biomass processing in green chemistry. Deep eutectic solvents provide a biodegradable, low-cost alternative with similar solvent capabilities for metal extraction, electrochemical applications, and organic synthesis. Both solvents reduce environmental impact by replacing volatile organic compounds in sustainable chemical processes.
Solvation and Extraction Abilities
Ionic liquids exhibit superior solvation capabilities due to their unique ionic structures, enabling them to dissolve a wide range of organic and inorganic compounds efficiently. Deep eutectic solvents (DES) offer tunable extraction abilities by adjusting their hydrogen bond donor and acceptor components, making them versatile for bioactive compound extraction. Your choice between ionic liquids and DES should consider target solute compatibility and environmental impact for optimal solvation and extraction performance.
Toxicity and Safety Profiles
Ionic liquids generally exhibit low volatility but can possess varying degrees of toxicity depending on their cation and anion composition, which may impact human health and environmental safety. Deep eutectic solvents (DES) are often considered safer and more biocompatible due to their natural component basis, resulting in reduced toxicity profiles in many applications. If you are selecting a solvent for sustainable and safe chemical processes, DES may offer a preferable safety profile compared to certain ionic liquids.
Cost and Commercial Availability
Ionic liquids generally have higher costs and limited commercial availability due to complex synthesis processes and expensive raw materials. Deep eutectic solvents offer a more cost-effective and scalable alternative, with easier preparation from readily available and inexpensive components. Your choice depends on balancing budget constraints with application-specific performance needs.
Future Perspectives and Research Trends
Future perspectives in ionic liquids emphasize their tunable physicochemical properties for green catalysis, energy storage, and pharmaceuticals, driving advances in sustainable chemical processes. Research trends in deep eutectic solvents focus on their low toxicity, biodegradability, and cost-effectiveness, promoting applications in biomass processing and CO2 capture. Emerging studies explore hybrid systems combining ionic liquids and deep eutectic solvents to optimize performance for environmental and industrial applications.
Ionic liquid vs deep eutectic solvent Infographic
 libmatt.com
libmatt.com