Atomic absorption spectroscopy (AAS) provides precise quantification of metal concentrations by measuring the absorption of light by free atoms, ideal for single-element analysis with high sensitivity. Inductively coupled plasma (ICP) techniques, including ICP-OES and ICP-MS, offer multi-element detection with greater dynamic range and lower detection limits, making them suitable for comprehensive elemental profiling in complex samples.
Table of Comparison
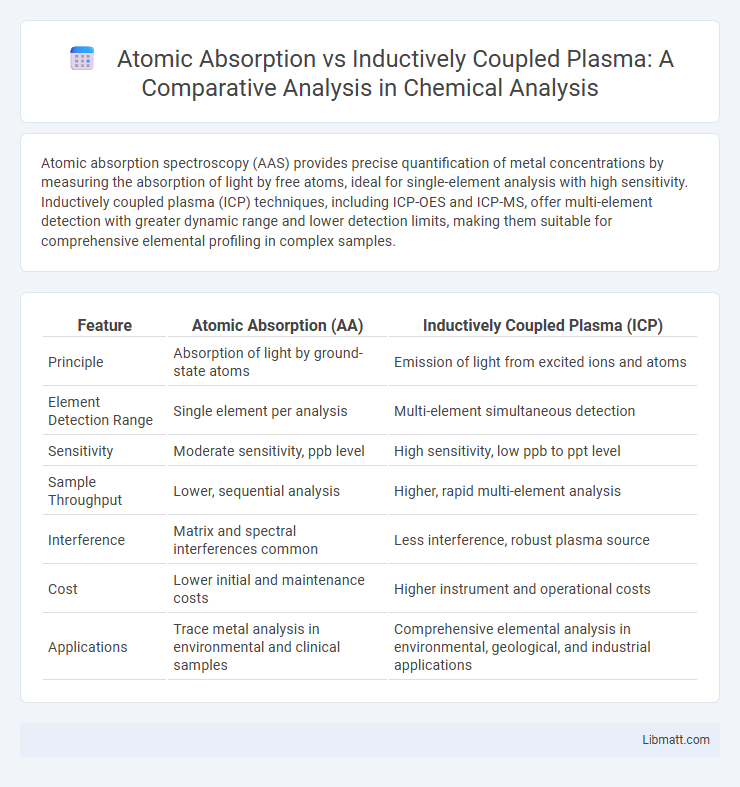
| Feature | Atomic Absorption (AA) | Inductively Coupled Plasma (ICP) |
|---|---|---|
| Principle | Absorption of light by ground-state atoms | Emission of light from excited ions and atoms |
| Element Detection Range | Single element per analysis | Multi-element simultaneous detection |
| Sensitivity | Moderate sensitivity, ppb level | High sensitivity, low ppb to ppt level |
| Sample Throughput | Lower, sequential analysis | Higher, rapid multi-element analysis |
| Interference | Matrix and spectral interferences common | Less interference, robust plasma source |
| Cost | Lower initial and maintenance costs | Higher instrument and operational costs |
| Applications | Trace metal analysis in environmental and clinical samples | Comprehensive elemental analysis in environmental, geological, and industrial applications |
Introduction to Atomic Absorption and Inductively Coupled Plasma
Atomic Absorption Spectroscopy (AAS) measures the concentration of elements by detecting the absorption of light by free atoms in the gaseous state, primarily used for trace metal analysis. Inductively Coupled Plasma (ICP) techniques, including ICP-OES and ICP-MS, utilize a high-temperature plasma source to atomize and ionize samples, enabling multi-element detection with higher sensitivity and faster throughput. Both methods serve critical roles in analytical chemistry, but ICP offers broader elemental coverage and lower detection limits compared to AAS.
Fundamental Principles of Atomic Absorption Spectroscopy
Atomic Absorption Spectroscopy (AAS) operates on the principle of measuring the absorption of light by free atoms in the ground state, which are generated in a flame or graphite furnace. This technique quantifies elemental concentrations by detecting the amount of light absorbed at specific wavelengths corresponding to each element's unique atomic transitions. Your choice between AAS and inductively coupled plasma (ICP) depends on the required sensitivity and multi-element analysis capability, with AAS excelling in elemental specificity through its fundamental reliance on atomic absorption of light.
Core Concepts of Inductively Coupled Plasma Spectroscopy
Inductively Coupled Plasma (ICP) Spectroscopy utilizes a high-temperature plasma source to atomize and excite samples, offering multi-element detection with superior sensitivity compared to Atomic Absorption Spectroscopy (AAS). The ICP system generates a plasma at approximately 10,000 Kelvin, efficiently ionizing elements for emission measurement across a broad spectral range. This technology enables simultaneous analysis of trace metals with precision and lower detection limits, making it ideal for complex matrices in environmental and industrial applications.
Instrumentation and Setup Differences
Atomic absorption (AA) utilizes a hollow cathode lamp as the light source and a single-beam optical system to measure the absorption of specific wavelengths by free atoms in a flame, requiring simpler instrumentation and setup. Inductively coupled plasma (ICP) employs a high-temperature plasma source generated by an RF coil to excite atoms and ions, coupled with a high-resolution spectrometer or mass spectrometer for multi-element detection, resulting in more complex and larger instrumentation. The AA setup focuses on measuring absorption at discrete wavelengths, while ICP measures emitted light or ion counts across a broad spectral range with higher sensitivity and throughput.
Sample Preparation Methods
Atomic absorption spectroscopy (AAS) typically requires simpler sample preparation methods such as acid digestion or direct aspiration of liquid samples to measure metal concentrations. Inductively coupled plasma (ICP) techniques often involve more rigorous preparation including complete digestion using microwave-assisted acid digestion to ensure total dissolution of solid matrices for accurate multi-element analysis. Both methods demand careful removal of interferences, but ICP sample preparation is usually more complex due to its multi-element capability and need for homogenous solutions.
Sensitivity and Detection Limits Comparison
Atomic absorption spectroscopy (AAS) generally offers sensitivity suitable for detecting metals at parts per million (ppm) levels, while inductively coupled plasma (ICP) techniques, such as ICP-OES and ICP-MS, provide significantly lower detection limits, reaching parts per billion (ppb) and parts per trillion (ppt), respectively. ICP methods demonstrate superior sensitivity due to their high-energy plasma source, enabling more efficient atomization and excitation of elements compared to the flame or graphite furnace in AAS. If your analysis requires ultra-trace detection and high precision, ICP techniques are preferable for achieving more accurate and reliable quantification of elemental concentrations.
Accuracy and Precision in Analytical Results
Atomic absorption spectroscopy (AAS) offers high accuracy and precision for trace metal analysis with simple sample preparation and limited spectral interferences, making it suitable for routine quantification. Inductively coupled plasma (ICP) techniques, particularly ICP-OES and ICP-MS, provide superior multi-element sensitivity, lower detection limits, and greater precision due to stable plasma conditions and advanced ionization efficiency. ICP methods excel in producing reproducible analytical results across complex matrices, outperforming AAS in accuracy for ultra-trace and multi-element determinations.
Common Applications in Analytical Chemistry
Atomic absorption spectrometry (AAS) excels in trace metal analysis of environmental water, food, and biological samples due to its high sensitivity and specificity for elements like lead, cadmium, and arsenic. Inductively coupled plasma (ICP) techniques, including ICP-OES and ICP-MS, are preferred for multi-element analysis in complex matrices such as geological samples, pharmaceuticals, and industrial chemicals because of their broad elemental coverage and superior detection limits. Your choice between AAS and ICP depends on the required sensitivity, the number of elements to be analyzed, and sample throughput needs.
Advantages and Limitations of Each Technique
Atomic absorption spectroscopy (AAS) offers high sensitivity and selectivity for detecting specific metal elements with relatively low operational costs and straightforward sample preparation. However, AAS is limited by its inability to perform multi-element analysis simultaneously and generally has lower throughput compared to inductively coupled plasma (ICP) methods. ICP techniques provide rapid, multi-element detection with excellent sensitivity and a wide dynamic range, but your laboratory may face higher initial equipment costs and more complex maintenance requirements.
Choosing the Right Technique for Your Analysis
Atomic absorption spectroscopy (AAS) is ideal for analyzing trace metals with high sensitivity and specificity in simple matrices, offering cost-effective and easy-to-use instrumentation. Inductively coupled plasma (ICP) techniques, including ICP-OES and ICP-MS, provide multi-element detection, lower detection limits, and superior precision for complex samples requiring comprehensive elemental analysis. Selecting the right technique depends on factors such as sample complexity, required detection limits, throughput, and budget constraints to achieve accurate and reliable analytical results.
Atomic absorption vs inductively coupled plasma Infographic
 libmatt.com
libmatt.com