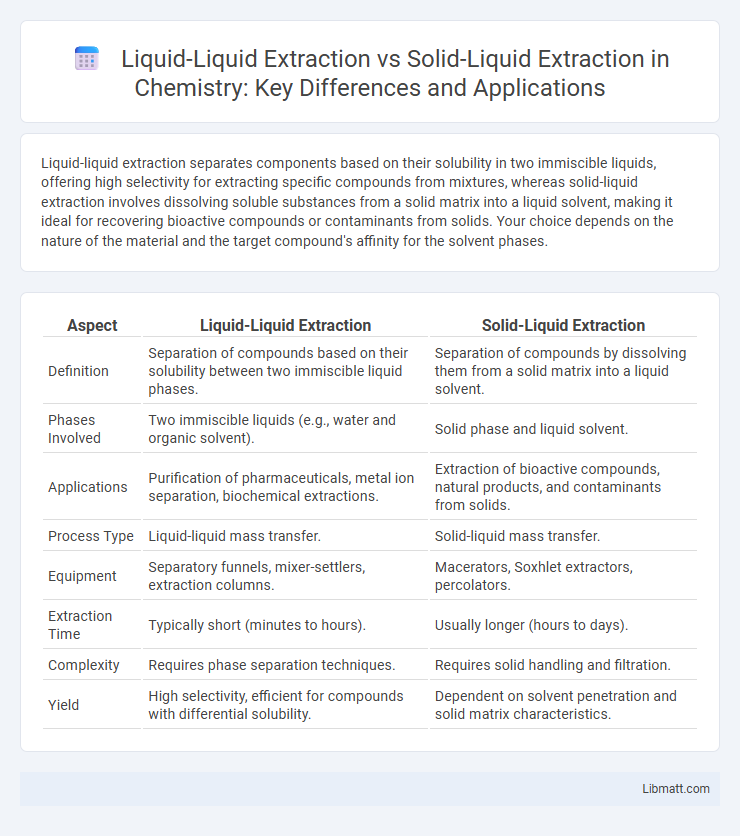
Liquid-liquid extraction separates components based on their solubility in two immiscible liquids, offering high selectivity for extracting specific compounds from mixtures, whereas solid-liquid extraction involves dissolving soluble substances from a solid matrix into a liquid solvent, making it ideal for recovering bioactive compounds or contaminants from solids. Your choice depends on the nature of the material and the target compound's affinity for the solvent phases.
Table of Comparison
| Aspect | Liquid-Liquid Extraction | Solid-Liquid Extraction |
|---|---|---|
| Definition | Separation of compounds based on their solubility between two immiscible liquid phases. | Separation of compounds by dissolving them from a solid matrix into a liquid solvent. |
| Phases Involved | Two immiscible liquids (e.g., water and organic solvent). | Solid phase and liquid solvent. |
| Applications | Purification of pharmaceuticals, metal ion separation, biochemical extractions. | Extraction of bioactive compounds, natural products, and contaminants from solids. |
| Process Type | Liquid-liquid mass transfer. | Solid-liquid mass transfer. |
| Equipment | Separatory funnels, mixer-settlers, extraction columns. | Macerators, Soxhlet extractors, percolators. |
| Extraction Time | Typically short (minutes to hours). | Usually longer (hours to days). |
| Complexity | Requires phase separation techniques. | Requires solid handling and filtration. |
| Yield | High selectivity, efficient for compounds with differential solubility. | Dependent on solvent penetration and solid matrix characteristics. |
Introduction to Extraction Techniques
Liquid-liquid extraction separates compounds based on their solubility differences between two immiscible liquids, commonly water and an organic solvent, enabling efficient purification of target molecules. Solid-liquid extraction involves dissolving components from a solid matrix into a suitable solvent, widely used in industries like pharmaceuticals and food processing for isolating bioactive substances. Understanding these extraction techniques helps optimize your process for maximum yield and purity according to the nature of the sample and desired compounds.
Principles of Liquid-Liquid Extraction
Liquid-liquid extraction operates on the principle of partitioning compounds between two immiscible liquid phases based on their relative solubilities and affinities, enabling efficient separation of target analytes. The process involves mixing an aqueous phase containing the solute with an organic solvent, where compounds distribute according to their partition coefficient. Your understanding of factors such as solvent selection, phase miscibility, and equilibrium is essential to optimize the extraction efficiency in liquid-liquid systems.
Principles of Solid-Liquid Extraction
Solid-liquid extraction involves the transfer of a solute from a solid matrix into a liquid solvent through diffusion and mass transfer processes. This technique relies on the solubility of the target compound in the solvent, particle size, agitation, and temperature to enhance extraction efficiency. Understanding these principles helps you optimize conditions for maximum yield in applications such as herbal extraction or pharmaceutical processing.
Comparison of Extraction Mechanisms
Liquid-liquid extraction involves the transfer of solutes between two immiscible liquid phases based on their differential solubility, relying heavily on partition coefficients and solvent affinity. Solid-liquid extraction operates through the diffusion of solutes from a solid matrix into a solvent, primarily driven by solubility and surface contact area. The key mechanistic distinction lies in liquid-liquid extraction's phase equilibrium between two liquids, whereas solid-liquid extraction depends on mass transfer from the solid to the liquid phase.
Solvent Selection Criteria
Solvent selection criteria for liquid-liquid extraction emphasize immiscibility with the other liquid phase, high selectivity for the target compound, low toxicity, and ease of separation after extraction. In solid-liquid extraction, solvents are chosen based on their ability to penetrate the solid matrix, dissolve the desired solutes effectively, and exhibit minimal interaction with impurities. Both methods require solvents with suitable polarity, boiling point, and chemical stability to optimize extraction efficiency and purity.
Efficiency and Yield Differences
Liquid-liquid extraction typically offers higher selectivity and purity, resulting in more efficient separation of compounds compared to solid-liquid extraction. Solid-liquid extraction often yields greater quantities of target compounds from solid matrices but may require longer processing times and additional purification steps. Efficiency differences depend on factors like solvent choice, solubility, and material properties, with liquid-liquid extraction favoring cleaner extracts and solid-liquid extraction maximizing overall yield from raw materials.
Applications in Industry and Research
Liquid-liquid extraction is widely used in the pharmaceutical and petrochemical industries for separating components based on solubility differences in immiscible liquids, enabling the purification of complex mixtures and recovery of valuable compounds. Solid-liquid extraction is essential in food technology and environmental analysis for isolating bioactive compounds and contaminants from solid matrices, facilitating quality control and pollutant monitoring. Your choice between these techniques depends on the nature of the sample and target analytes, with liquid-liquid extraction preferred for liquid samples and solid-liquid extraction suited for solid or semi-solid materials.
Advantages and Limitations
Liquid-liquid extraction offers high selectivity and efficiency in separating compounds due to differences in solubility between two immiscible liquids, making it ideal for complex mixtures. However, it can be limited by emulsion formation, solvent toxicity, and the need for solvent disposal, which may impact environmental and operational costs. Solid-liquid extraction provides a simpler, cost-effective method for extracting bioactive compounds from solid matrices, but often requires longer processing times and may yield lower purity compared to liquid-liquid extraction.
Environmental and Safety Considerations
Liquid-liquid extraction often involves large volumes of organic solvents that can be hazardous and pose environmental risks due to volatility and toxicity. Solid-liquid extraction typically uses less solvent, reducing the potential for environmental contamination and improving safety for your laboratory personnel. Selecting solid-liquid extraction methods can minimize chemical waste and exposure, aligning with greener and safer operational practices.
Choosing the Appropriate Extraction Method
Choosing the appropriate extraction method depends on the nature of the material and desired compounds: liquid-liquid extraction works best for separating components between two immiscible liquids, particularly when targeting analytes soluble in different solvents. Solid-liquid extraction is more suitable for extracting compounds from solid matrices by using a solvent to dissolve the target substances, commonly applied in food, pharmaceuticals, and environmental samples. Your selection should consider factors such as solubility, extraction efficiency, and sample physical state to optimize yield and purity.
Liquid-liquid extraction vs solid-liquid extraction Infographic
 libmatt.com
libmatt.com