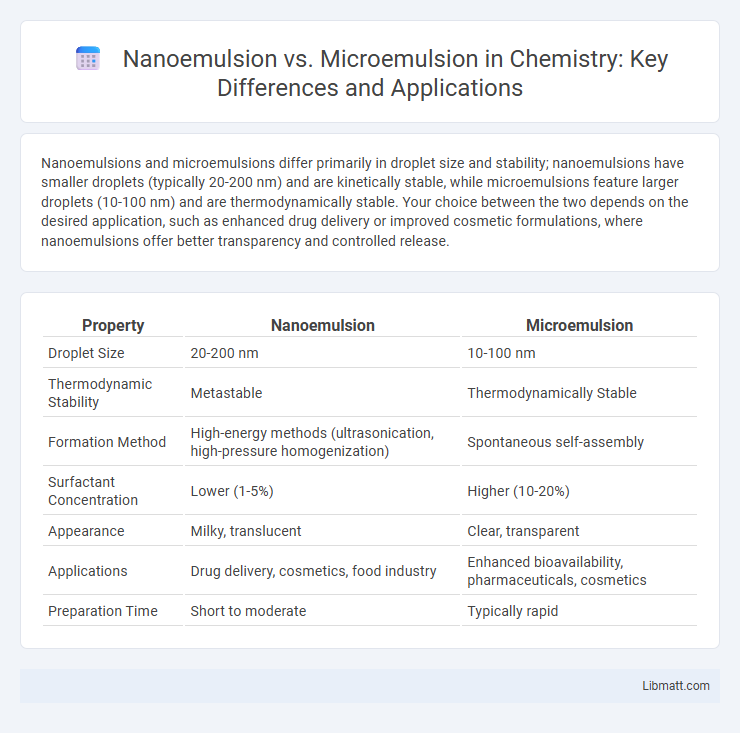
Nanoemulsions and microemulsions differ primarily in droplet size and stability; nanoemulsions have smaller droplets (typically 20-200 nm) and are kinetically stable, while microemulsions feature larger droplets (10-100 nm) and are thermodynamically stable. Your choice between the two depends on the desired application, such as enhanced drug delivery or improved cosmetic formulations, where nanoemulsions offer better transparency and controlled release.
Table of Comparison
| Property | Nanoemulsion | Microemulsion |
|---|---|---|
| Droplet Size | 20-200 nm | 10-100 nm |
| Thermodynamic Stability | Metastable | Thermodynamically Stable |
| Formation Method | High-energy methods (ultrasonication, high-pressure homogenization) | Spontaneous self-assembly |
| Surfactant Concentration | Lower (1-5%) | Higher (10-20%) |
| Appearance | Milky, translucent | Clear, transparent |
| Applications | Drug delivery, cosmetics, food industry | Enhanced bioavailability, pharmaceuticals, cosmetics |
| Preparation Time | Short to moderate | Typically rapid |
Introduction to Nanoemulsions and Microemulsions
Nanoemulsions and microemulsions are both advanced colloidal delivery systems characterized by their droplet sizes, with nanoemulsions typically ranging from 20 to 200 nanometers and microemulsions from 10 to 100 nanometers. Nanoemulsions are kinetically stable, meaning they require external energy for formation and maintain their structure over time, while microemulsions are thermodynamically stable and form spontaneously due to their specific surfactant and co-surfactant compositions. Understanding these key differences helps optimize Your formulation choices in pharmaceuticals, cosmetics, and food industries for enhanced bioavailability and stability.
Key Differences in Structure and Composition
Nanoemulsions consist of oil and water droplets with sizes typically ranging from 20 to 200 nanometers, stabilized by surfactants but remaining thermodynamically unstable, leading to eventual phase separation. Microemulsions feature droplet sizes generally between 10 to 100 nanometers and form spontaneous, thermodynamically stable mixtures due to the presence of a balanced combination of oil, water, and high concentrations of surfactants and co-surfactants. The distinct stability, droplet size range, and surfactant composition define their functional and practical applications in pharmaceuticals, cosmetics, and food industries.
Particle Size and Stability Comparisons
Nanoemulsions typically exhibit particle sizes ranging from 20 to 200 nanometers, resulting in greater stability against gravitational separation and coalescence compared to microemulsions, which have slightly larger particles between 100 and 500 nanometers. The smaller droplet size in nanoemulsions enhances kinetic stability due to lower sedimentation rates and reduced Brownian motion effects, whereas microemulsions rely on thermodynamic stability driven by surfactant concentration and composition. Consequently, nanoemulsions maintain uniform dispersions longer under varying environmental conditions, while microemulsions achieve stability predominantly through their equilibrium phase behavior.
Preparation Methods: Nanoemulsion vs Microemulsion
Nanoemulsions are typically prepared using high-energy methods such as ultrasonic emulsification or high-pressure homogenization, which create small droplet sizes ranging from 20 to 200 nm through intense mechanical forces. Microemulsions form spontaneously due to thermodynamic stability and require careful selection and mixing of surfactants, co-surfactants, oil, and water phases, resulting in droplet sizes between 10 and 100 nm. The preparation of nanoemulsions demands external energy input, whereas microemulsions rely on the intrinsic amphiphilic properties of their components to self-assemble.
Mechanisms of Formation
Nanoemulsions form through high-energy methods like ultrasonication or high-pressure homogenization, which reduce droplet size to 20-200 nm by creating intense shear forces and turbulence. Microemulsions spontaneously form via low-energy processes due to thermodynamic stability, relying on precise surfactant and co-surfactant balance to minimize interfacial tension and achieve droplet sizes typically between 10-100 nm. The key difference lies in nanoemulsions requiring external energy input for kinetic stability, whereas microemulsions achieve thermodynamically stable structures through optimized surfactant chemistry.
Applications in Pharmaceuticals and Cosmetics
Nanoemulsions and microemulsions both enhance drug delivery and cosmetic formulations by improving solubility and bioavailability of active ingredients; nanoemulsions offer superior stability and deeper skin penetration due to their smaller droplet size (20-200 nm) compared to microemulsions (10-100 nm). Pharmaceuticals utilize nanoemulsions for targeted drug delivery, controlled release, and enhanced absorption, while microemulsions are favored for their ease of preparation and thermodynamic stability in topical formulations. Your choice between the two depends on the specific application, desired stability, and release profile required in therapeutic or cosmetic products.
Advantages and Limitations
Nanoemulsions offer advantages such as enhanced bioavailability, improved stability, and smaller droplet sizes ranging from 20 to 200 nm, which facilitate targeted drug delivery and increased absorption rates. Limitations include complex manufacturing processes and potential instability under certain environmental conditions, such as temperature and pH variations. In contrast, microemulsions exhibit thermodynamic stability and ease of formulation but have larger droplet sizes (typically 100 to 500 nm) that may limit their penetration efficiency and can cause skin irritation due to higher surfactant concentrations.
Safety and Toxicity Profiles
Nanoemulsions generally exhibit enhanced safety and lower toxicity due to their smaller droplet size, which enables more stable and controlled release of encapsulated substances. Microemulsions, while thermodynamically stable, often contain higher concentrations of surfactants and co-surfactants that may increase cytotoxicity and skin irritation risks. Your formulation choices should consider these differences to optimize biocompatibility and minimize adverse effects in pharmaceutical or cosmetic applications.
Market Trends and Future Prospects
The nanoemulsion market is experiencing rapid growth due to its superior stability, smaller droplet size (20-200 nm), and enhanced bioavailability, making it highly favored in pharmaceuticals, cosmetics, and food industries. In contrast, microemulsions (droplet size 100-500 nm) maintain a steady demand driven by their thermodynamic stability and ease of formulation, especially in enhanced oil recovery and topical drug delivery. Your investment in nanoemulsion technology could capitalize on expanding applications and increasing consumer demand for innovative, efficient delivery systems in emerging markets.
Conclusion: Choosing Between Nanoemulsions and Microemulsions
Nanoemulsions offer superior kinetic stability and smaller droplet size, making them ideal for enhancing bioavailability in pharmaceuticals and cosmetics. Microemulsions exhibit thermodynamic stability and ease of formulation, suitable for applications requiring long-term shelf life and spontaneous formation. Selecting between nanoemulsions and microemulsions depends on the specific needs for stability, droplet size, and preparation complexity in the target application.
nanoemulsion vs microemulsion Infographic
 libmatt.com
libmatt.com