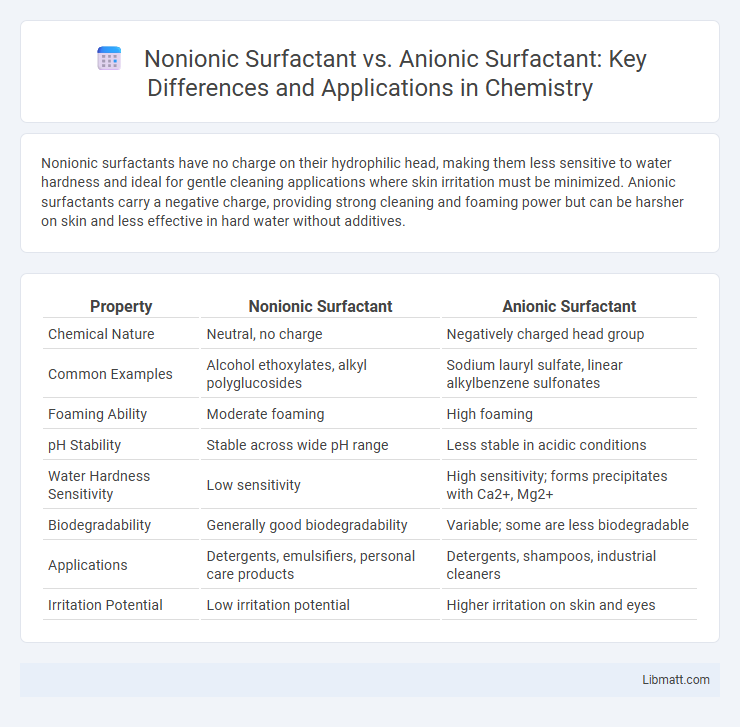
Nonionic surfactants have no charge on their hydrophilic head, making them less sensitive to water hardness and ideal for gentle cleaning applications where skin irritation must be minimized. Anionic surfactants carry a negative charge, providing strong cleaning and foaming power but can be harsher on skin and less effective in hard water without additives.
Table of Comparison
| Property | Nonionic Surfactant | Anionic Surfactant |
|---|---|---|
| Chemical Nature | Neutral, no charge | Negatively charged head group |
| Common Examples | Alcohol ethoxylates, alkyl polyglucosides | Sodium lauryl sulfate, linear alkylbenzene sulfonates |
| Foaming Ability | Moderate foaming | High foaming |
| pH Stability | Stable across wide pH range | Less stable in acidic conditions |
| Water Hardness Sensitivity | Low sensitivity | High sensitivity; forms precipitates with Ca2+, Mg2+ |
| Biodegradability | Generally good biodegradability | Variable; some are less biodegradable |
| Applications | Detergents, emulsifiers, personal care products | Detergents, shampoos, industrial cleaners |
| Irritation Potential | Low irritation potential | Higher irritation on skin and eyes |
Introduction to Surfactants
Nonionic surfactants lack charged head groups, making them less sensitive to water hardness and ideal for use in delicate formulations like cosmetics and pharmaceuticals. Anionic surfactants possess negatively charged head groups that provide strong cleansing and foaming properties, commonly used in household detergents and industrial cleaners. Understanding the differences helps you choose the right surfactant for effective cleaning and formulation stability.
Defining Nonionic and Anionic Surfactants
Nonionic surfactants are surface-active agents that do not carry any electrical charge, making them less sensitive to water hardness and suitable for delicate formulations. Anionic surfactants possess a negatively charged hydrophilic head, providing strong cleaning and foaming properties, particularly effective in removing oily soils. Your choice between these surfactants depends on application requirements such as detergent strength, mildness, and compatibility with other ingredients.
Molecular Structure Differences
Nonionic surfactants feature hydrophilic groups without charge, typically ethylene oxide chains, while anionic surfactants contain negatively charged head groups such as sulfate or carboxylate ions. This molecular structure difference affects solubility, foaming properties, and interactions with water hardness ions. Understanding these distinctions helps you select the right surfactant for formulations needing specific cleaning efficiency or mildness.
Mechanisms of Action
Nonionic surfactants reduce surface tension by disrupting lipid-lipid interactions without generating charged species, allowing gentle cleaning and emulsification in sensitive formulations. Anionic surfactants function through negatively charged head groups that bind to positively charged dirt and oils, providing strong detergency and foaming capabilities. Your choice depends on the desired balance between mildness and cleaning power, with nonionic surfactants suited for delicate applications and anionic surfactants ideal for heavy-duty cleansing.
Key Applications in Industry
Nonionic surfactants are widely used in industries such as pharmaceuticals, cosmetics, and food processing for their mildness and compatibility with sensitive formulations, making them ideal for emulsification, detergency, and solubilization. Anionic surfactants dominate in household cleaning products, textile processing, and oil recovery due to their strong foaming properties and effective removal of dirt and grease. Understanding the specific applications of these surfactants can help you select the right type for your industrial needs, optimizing performance and product quality.
Solubility and Compatibility
Nonionic surfactants exhibit superior solubility in a wide range of solvents, including both polar and non-polar environments, due to their lack of charge, enhancing their compatibility with various formulations. Anionic surfactants, carrying a negatively charged head group, demonstrate high solubility in aqueous solutions but are less compatible with cationic ingredients and hard water, which can reduce their effectiveness. The neutral charge of nonionic surfactants allows them to maintain stability and performance in diverse pH levels and ionic strengths, making them more versatile in complex mixtures compared to anionic surfactants.
Environmental Impact and Biodegradability
Nonionic surfactants generally exhibit higher biodegradability and lower aquatic toxicity compared to anionic surfactants, making them more environmentally friendly for widespread use. Anionic surfactants, often derived from petrochemicals, tend to persist longer in aquatic environments and can disrupt aquatic ecosystems due to their higher toxicity to fish and invertebrates. Selecting nonionic surfactants can reduce the environmental impact of detergents and cleaning agents by promoting faster breakdown and lower bioaccumulation in water bodies.
Advantages and Disadvantages
Nonionic surfactants offer advantages such as low toxicity, excellent compatibility with a wide range of chemicals, and superior performance in hard water conditions, making them ideal for sensitive skin formulations and cleaning products. Anionic surfactants provide strong foaming and effective dirt removal but can cause skin irritation and lose efficiency in the presence of hard water due to interactions with calcium and magnesium ions. Your choice depends on whether you prioritize gentleness and chemical stability (nonionic) or powerful cleansing and foaming action (anionic).
Choosing the Right Surfactant for Your Needs
Selecting the right surfactant depends on the application and desired properties; nonionic surfactants offer excellent compatibility with various ingredients and perform well in hard water, making them ideal for mild cleansing and cosmetic products. Anionic surfactants provide strong detergency and foaming characteristics, suitable for heavy-duty cleaning and industrial use. Understanding the balance between gentle cleaning power and effective grease removal helps determine whether a nonionic or anionic surfactant is best for your formulation.
Future Trends in Surfactant Development
Nonionic surfactants are favored for their mildness and biodegradability, driving future trends toward eco-friendly formulations in personal care and household products. Anionic surfactants continue evolving with enhanced foaming and cleaning efficiency, supporting more sustainable and high-performance detergent applications. Your choice of surfactant will increasingly rely on balancing environmental impact with functional performance as innovation accelerates.
Nonionic surfactant vs anionic surfactant Infographic
 libmatt.com
libmatt.com