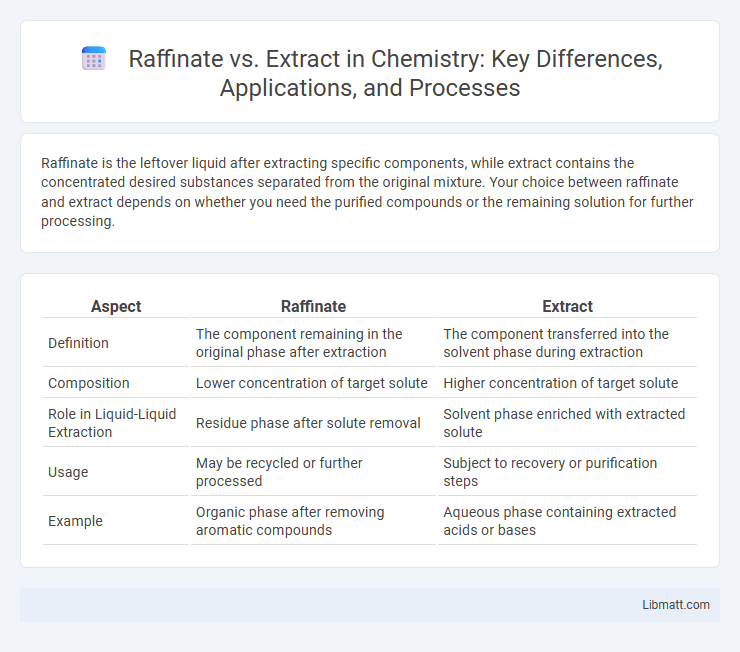
Raffinate is the leftover liquid after extracting specific components, while extract contains the concentrated desired substances separated from the original mixture. Your choice between raffinate and extract depends on whether you need the purified compounds or the remaining solution for further processing.
Table of Comparison
| Aspect | Raffinate | Extract |
|---|---|---|
| Definition | The component remaining in the original phase after extraction | The component transferred into the solvent phase during extraction |
| Composition | Lower concentration of target solute | Higher concentration of target solute |
| Role in Liquid-Liquid Extraction | Residue phase after solute removal | Solvent phase enriched with extracted solute |
| Usage | May be recycled or further processed | Subject to recovery or purification steps |
| Example | Organic phase after removing aromatic compounds | Aqueous phase containing extracted acids or bases |
Introduction to Raffinate and Extract
Raffinate and extract are two key products in liquid-liquid extraction processes, where a solvent selectively separates components from a feed mixture. Raffinate refers to the phase that remains after the target components have been removed, typically containing impurities or less desirable substances. Your choice between raffinate and extract depends on the desired purity and recovery of the target compound in industrial applications.
Understanding Raffinate: Definition and Properties
Raffinate refers to the portion of a liquid mixture remaining after selective extraction of one or more components, usually containing the undesired or less valuable substances. Its properties depend on the efficiency of the extraction process, solvent selection, and feed composition, typically characterized by lower concentrations of the extracted solute. Understanding the chemical composition and physical properties of raffinate is essential for optimizing downstream processing and improving overall separation performance.
What is Extract? Key Characteristics
Extract is the portion of a mixture obtained after solvent extraction that contains the desired solute, typically concentrated and rich in target compounds. Key characteristics of extract include high purity, increased concentration of specific components, and reduced impurities compared to the original feed. Extract's quality and composition depend on solvent selectivity, extraction conditions, and solute-solvent affinity.
Industrial Applications of Raffinate and Extract
Raffinate and extract streams are critical in industrial solvent extraction processes, where raffinate typically contains the non-target components and extract holds the desired solute. Industries such as petrochemical refining leverage raffinate to remove impurities from feedstocks, while extract is purified to isolate valuable compounds like metals or organic solvents. Your operations benefit from optimizing the separation efficiency to improve product quality and reduce downstream processing costs.
Separation Processes: How Raffinate and Extract are Obtained
In separation processes such as liquid-liquid extraction, the feed mixture is divided into two immiscible phases: the raffinate and the extract. The raffinate is the phase that remains depleted of the target solute, while the extract phase contains the concentrated solute dissolved in the extracting solvent. Factors like distribution coefficient, solvent selectivity, and equilibrium stage design critically determine the efficiency of obtaining high-purity raffinate and extract streams.
Chemical Composition Differences
Raffinate and extract streams differ significantly in chemical composition due to the selective removal of target components during liquid-liquid extraction. Raffinate primarily contains the non-extracted components with reduced concentrations of solutes, while the extract is rich in the solutes transferred from the feed, typically featuring higher purity of the desired chemical species. Understanding these compositional differences is essential for optimizing separation efficiency and downstream processing.
Factors Influencing Raffinate and Extract Purity
Raffinate and extract purity are primarily influenced by factors such as feed composition, solvent selectivity, and operating conditions like temperature and pressure in liquid-liquid extraction processes. The distribution coefficient directly impacts how well components separate, affecting the concentration of impurities in both the raffinate and extract streams. Optimizing these parameters ensures your process achieves the desired purity levels for efficient downstream operations.
Advantages and Disadvantages of Raffinate vs Extract
Raffinate offers the advantage of containing fewer impurities and is typically easier to handle due to its lower concentration of the targeted component, making it beneficial for further processing or disposal. Extract often has a higher concentration of the desired substance, enabling more efficient recovery but may require additional purification steps and involves handling of potentially hazardous solvents. Balancing the trade-offs between processing complexity and concentration purity is crucial when choosing between raffinate and extract in separation processes.
Environmental Impact and Handling Considerations
Raffinate streams generally contain fewer hazardous components compared to extract streams, reducing the potential environmental risks associated with disposal or accidental release. Extract phases often contain concentrated solvents or pollutants requiring specialized handling, treatment, and disposal methods to minimize ecological harm. Proper management of both raffinate and extract is critical for compliance with environmental regulations and for mitigating soil and water contamination risks.
Raffinate vs Extract: Key Takeaways and Future Perspectives
Raffinate refers to the remaining liquid phase after extraction, typically depleted of the targeted solutes, while extract is the phase enriched with desired components separated from the feed mixture. Advances in solvent selection, membrane technology, and process optimization are driving improvements in separation efficiency and sustainability for both raffinate and extract streams. Future perspectives emphasize integrating artificial intelligence and green solvents to enhance selectivity and reduce environmental impact in industrial extraction processes.
Raffinate vs extract Infographic
 libmatt.com
libmatt.com