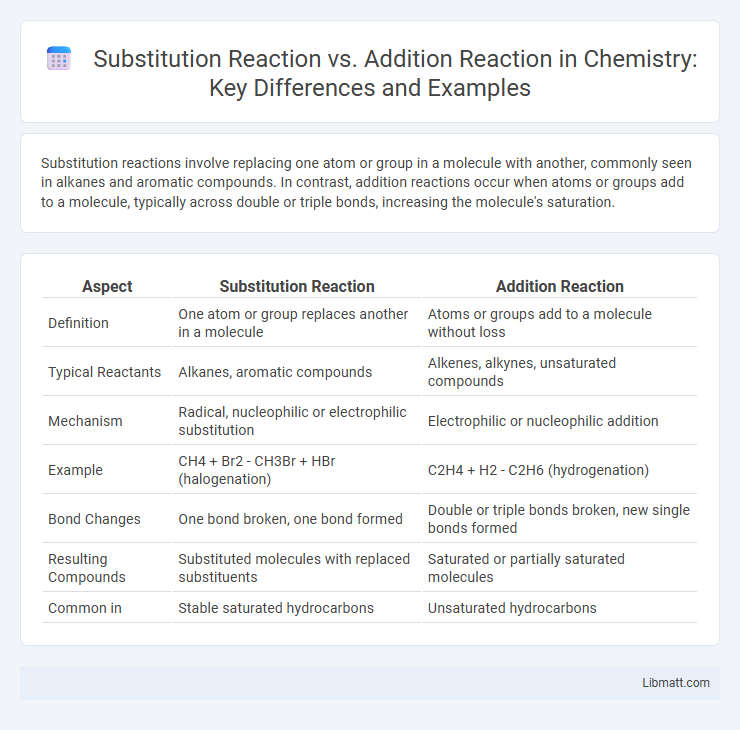
Substitution reactions involve replacing one atom or group in a molecule with another, commonly seen in alkanes and aromatic compounds. In contrast, addition reactions occur when atoms or groups add to a molecule, typically across double or triple bonds, increasing the molecule's saturation.
Table of Comparison
| Aspect | Substitution Reaction | Addition Reaction |
|---|---|---|
| Definition | One atom or group replaces another in a molecule | Atoms or groups add to a molecule without loss |
| Typical Reactants | Alkanes, aromatic compounds | Alkenes, alkynes, unsaturated compounds |
| Mechanism | Radical, nucleophilic or electrophilic substitution | Electrophilic or nucleophilic addition |
| Example | CH4 + Br2 - CH3Br + HBr (halogenation) | C2H4 + H2 - C2H6 (hydrogenation) |
| Bond Changes | One bond broken, one bond formed | Double or triple bonds broken, new single bonds formed |
| Resulting Compounds | Substituted molecules with replaced substituents | Saturated or partially saturated molecules |
| Common in | Stable saturated hydrocarbons | Unsaturated hydrocarbons |
Introduction to Substitution and Addition Reactions
Substitution reactions involve the replacement of an atom or group in a molecule with a different atom or group, commonly seen in organic chemistry with halogenated compounds and nucleophiles. Addition reactions occur when two reactants combine to form a single product without the loss of atoms, typically involving alkenes or alkynes where pi bonds are converted to sigma bonds. Both reaction types are fundamental in synthesizing diverse organic compounds, influencing mechanisms such as nucleophilic substitution (SN1, SN2) and electrophilic addition.
Defining Substitution Reactions
Substitution reactions involve the replacement of an atom or group in a molecule with another atom or group, often occurring in organic compounds like alkanes and aromatic rings. These reactions differ significantly from addition reactions, which involve the addition of atoms or groups to a molecule without losing any original components. Understanding substitution reactions helps you predict products and mechanisms, especially in nucleophilic or electrophilic substitution processes.
Understanding Addition Reactions
Addition reactions involve the combination of two or more reactants to form a single product, typically occurring in unsaturated organic compounds such as alkenes and alkynes. These reactions increase the saturation of the molecule by breaking double or triple bonds and adding atoms or groups across them. Understanding the mechanism of addition reactions is crucial for predicting product formation and reactivity in synthetic organic chemistry.
Key Differences Between Substitution and Addition
Substitution reactions involve replacing one atom or group in a molecule with another, often preserving the overall number of atoms, while addition reactions result in the joining of two molecules or atoms, increasing the molecular size without eliminating any part. In substitution, typically a single bond is broken and a new bond is formed at the same carbon atom, whereas addition reactions break multiple bonds (usually double or triple bonds) and add atoms directly to the original molecule. Understanding these key differences helps you predict reaction pathways and mechanisms critical in organic synthesis and industrial chemistry.
Mechanisms of Substitution Reactions
Substitution reactions involve the replacement of an atom or group of atoms in a molecule by another atom or group, typically proceeding via nucleophilic or electrophilic pathways. The mechanisms include SN1, characterized by a two-step process with carbocation intermediate formation leading to racemization, and SN2, a one-step concerted reaction where the nucleophile attacks from the opposite side causing inversion of stereochemistry. Electrophilic substitution commonly occurs in aromatic compounds through an intermediate sigma complex, stabilizing the transition state before restoring aromaticity.
Mechanisms of Addition Reactions
Addition reactions proceed through mechanisms involving the attack of electrophiles or nucleophiles on multiple bonds, such as alkenes or alkynes, resulting in the formation of new single bonds. These mechanisms often include carbocation intermediates in electrophilic addition or concerted steps in pericyclic additions, increasing the molecule's saturation. Understanding how your reactants interact during electrophilic or nucleophilic addition can help predict product formation and reaction outcomes.
Real-Life Examples of Substitution Reactions
Substitution reactions involve the replacement of an atom or group in a molecule, commonly seen in pharmaceuticals like the synthesis of analgesics such as paracetamol, where hydroxyl groups substitute hydrogen atoms. In agriculture, herbicides like 2,4-D are produced through substitution reactions to target specific plant enzymes. Your understanding of these real-life examples highlights the importance of substitution reactions in developing essential compounds for medicine and crop protection.
Real-Life Examples of Addition Reactions
Addition reactions are commonly observed in the industrial synthesis of polymers, such as polyethylene and polypropylene, produced via the addition of ethylene or propylene monomers. In pharmaceuticals, addition reactions facilitate the formation of drug intermediates through the addition of halogens to alkenes, enhancing molecular complexity. The manufacture of alcohols through the hydration of alkenes exemplifies another practical application of addition reactions in chemical industries.
Factors Influencing Each Type of Reaction
Substitution reactions are influenced by factors such as the nature of the leaving group, the stability of the carbocation intermediate, and the strength of the nucleophile, which determine the reaction mechanism (SN1 or SN2). Addition reactions depend primarily on the presence of a double or triple bond, the electrophilicity of the substrate, and reaction conditions like temperature and catalyst presence. Understanding these factors helps you predict whether a substitution or addition reaction will occur under specific chemical environments.
Applications in Industry and Organic Synthesis
Substitution reactions are pivotal in pharmaceuticals for modifying aromatic compounds and synthesizing intermediates like halogenated drugs, enhancing drug efficacy and specificity. Addition reactions are extensively employed in polymer industry to produce plastics such as polyethylene and polypropylene through polymerization of alkenes, offering materials with diverse properties. Both reactions enable tailored molecular modifications essential for producing agrochemicals, dyes, and specialty chemicals in industrial organic synthesis.
Substitution reaction vs addition reaction Infographic
 libmatt.com
libmatt.com