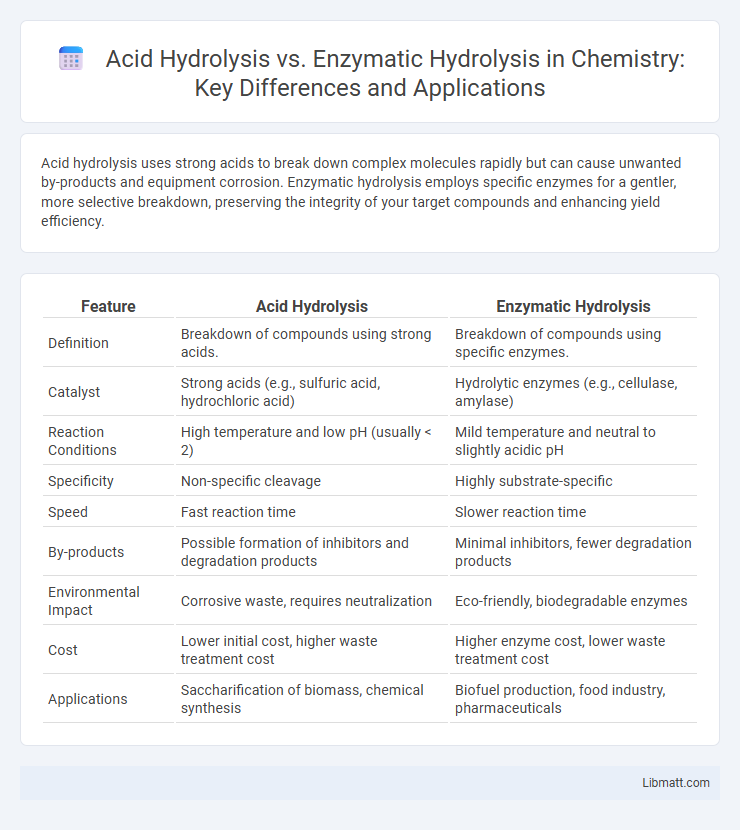
Acid hydrolysis uses strong acids to break down complex molecules rapidly but can cause unwanted by-products and equipment corrosion. Enzymatic hydrolysis employs specific enzymes for a gentler, more selective breakdown, preserving the integrity of your target compounds and enhancing yield efficiency.
Table of Comparison
| Feature | Acid Hydrolysis | Enzymatic Hydrolysis |
|---|---|---|
| Definition | Breakdown of compounds using strong acids. | Breakdown of compounds using specific enzymes. |
| Catalyst | Strong acids (e.g., sulfuric acid, hydrochloric acid) | Hydrolytic enzymes (e.g., cellulase, amylase) |
| Reaction Conditions | High temperature and low pH (usually < 2) | Mild temperature and neutral to slightly acidic pH |
| Specificity | Non-specific cleavage | Highly substrate-specific |
| Speed | Fast reaction time | Slower reaction time |
| By-products | Possible formation of inhibitors and degradation products | Minimal inhibitors, fewer degradation products |
| Environmental Impact | Corrosive waste, requires neutralization | Eco-friendly, biodegradable enzymes |
| Cost | Lower initial cost, higher waste treatment cost | Higher enzyme cost, lower waste treatment cost |
| Applications | Saccharification of biomass, chemical synthesis | Biofuel production, food industry, pharmaceuticals |
Introduction to Hydrolysis Methods
Acid hydrolysis employs strong acids such as sulfuric acid to break down complex carbohydrates into simple sugars by cleaving glycosidic bonds under high temperature and pressure conditions. Enzymatic hydrolysis uses specific enzymes like cellulase and hemicellulase to catalyze the breakdown of polysaccharides into fermentable sugars at milder temperatures and neutral pH. The choice between acid and enzymatic hydrolysis depends on factors including substrate type, process efficiency, product purity, and environmental impact.
Overview of Acid Hydrolysis
Acid hydrolysis involves breaking down complex carbohydrates into simple sugars using strong acids like sulfuric or hydrochloric acid under high temperature and pressure conditions. This method is highly effective for converting lignocellulosic biomass into fermentable sugars but can lead to the formation of inhibitory by-products such as furfurals and weak acids. Acid hydrolysis is widely used in industrial biomass processing due to its rapid reaction rates, although it requires corrosion-resistant equipment and careful handling.
Overview of Enzymatic Hydrolysis
Enzymatic hydrolysis uses specific enzymes like cellulases and hemicellulases to break down complex carbohydrates into simple sugars under mild conditions, preserving sugar integrity and reducing byproduct formation. This process is highly selective, environmentally friendly, and energy-efficient compared to acid hydrolysis, which often involves harsh acids and high temperatures that can degrade sugars. Enzymatic hydrolysis is widely applied in biofuel production, food processing, and biomass conversion due to its high specificity and ability to maximize sugar yield from lignocellulosic materials.
Mechanisms of Acid vs Enzymatic Hydrolysis
Acid hydrolysis involves breaking down complex carbohydrates by protonating glycosidic oxygen atoms, leading to cleavage of glycosidic bonds under highly acidic conditions and elevated temperatures. Enzymatic hydrolysis employs specific enzymes such as cellulases or amylases which selectively catalyze the hydrolysis of glycosidic bonds via substrate binding and catalytic residues, operating under mild physiological conditions. The acid hydrolysis mechanism is non-specific and often causes sugar degradation, while enzymatic hydrolysis offers higher specificity and preserves sugar integrity through controlled biochemical reactions.
Efficiency and Yield Comparison
Enzymatic hydrolysis typically offers higher selectivity and yields in breaking down biomass into fermentable sugars compared to acid hydrolysis, which can cause sugar degradation and formation of inhibitory byproducts. Acid hydrolysis is faster but often requires harsher conditions, leading to lower overall sugar recovery and potential equipment corrosion. Enzymatic processes, though slower and costlier, achieve greater efficiency in preserving sugar integrity, resulting in higher fermentable sugar yield and improved downstream bioproduct conversion.
Substrate Specificity Differences
Acid hydrolysis exhibits broad substrate specificity, effectively breaking down a wide range of polysaccharides such as cellulose, hemicellulose, and starch into simpler sugars, but often leads to the formation of inhibitory by-products. Enzymatic hydrolysis demonstrates high substrate specificity, targeting particular glycosidic bonds with enzymes like cellulases, hemicellulases, and amylases to yield sugars with minimal side reactions. This specificity results in more efficient conversion processes, preserving sugar integrity and reducing the need for extensive downstream purification.
Environmental Impact and Safety
Acid hydrolysis generates hazardous waste and corrosive byproducts, posing significant environmental disposal challenges and safety risks to workers due to chemical exposure. Enzymatic hydrolysis operates under milder conditions with biodegradable enzymes, minimizing toxic waste and reducing the ecological footprint. The enzymatic process enhances workplace safety by eliminating the need for harsh chemicals, leading to a more sustainable and safer bioconversion method.
Industrial Applications
Acid hydrolysis is widely used in the industrial production of bioethanol and the breakdown of lignocellulosic biomass due to its rapid and cost-effective cleavage of polysaccharides into fermentable sugars. Enzymatic hydrolysis offers higher specificity and milder reaction conditions, making it essential in the pharmaceutical industry for producing high-purity oligosaccharides and in food processing for modifying starches. Both methods are critical in biomass conversion technologies, with acid hydrolysis favoring large-scale, low-cost operations and enzymatic hydrolysis preferred for sustainability and product quality.
Cost Considerations
Acid hydrolysis generally incurs higher operational costs due to the need for corrosion-resistant equipment and extensive neutralization steps, increasing both capital and maintenance expenses. Enzymatic hydrolysis, while often more expensive upfront because of enzyme production costs, offers lower energy requirements and reduced equipment wear, leading to long-term cost savings. Your choice should weigh these economic factors against the scale and specific application of hydrolysis processes.
Conclusion: Choosing the Optimal Hydrolysis Method
Acid hydrolysis offers rapid breakdown of complex carbohydrates but risks sugar degradation and formation of inhibitory compounds, while enzymatic hydrolysis provides higher specificity and milder conditions with fewer byproducts. The optimal hydrolysis method depends on the substrate type, desired product purity, and economic considerations such as enzyme cost versus acid handling expenses. Industrial applications favor enzymatic hydrolysis for biofuel and biochemical production due to its efficiency and sustainability, despite longer processing times.
Acid hydrolysis vs enzymatic hydrolysis Infographic
 libmatt.com
libmatt.com