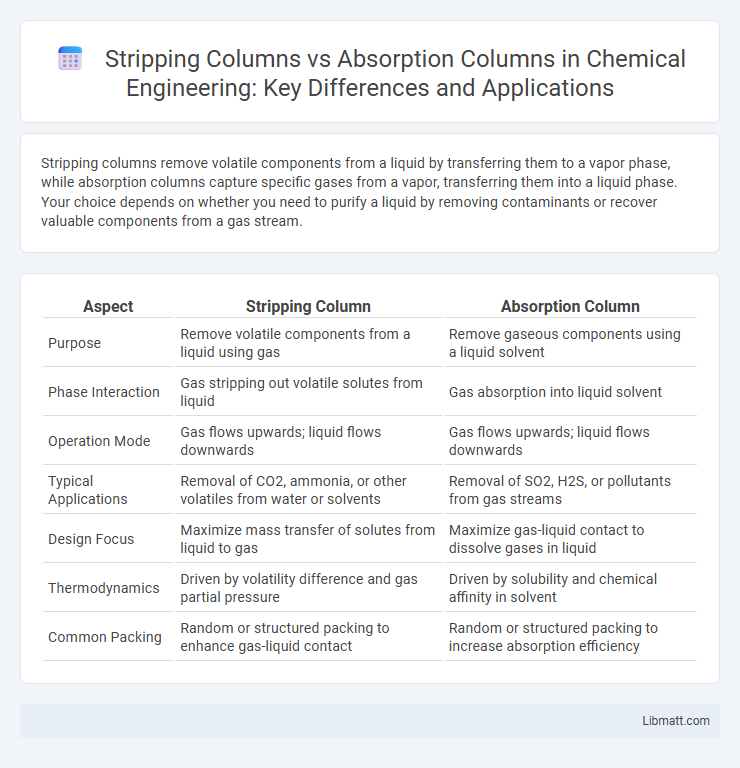
Stripping columns remove volatile components from a liquid by transferring them to a vapor phase, while absorption columns capture specific gases from a vapor, transferring them into a liquid phase. Your choice depends on whether you need to purify a liquid by removing contaminants or recover valuable components from a gas stream.
Table of Comparison
| Aspect | Stripping Column | Absorption Column |
|---|---|---|
| Purpose | Remove volatile components from a liquid using gas | Remove gaseous components using a liquid solvent |
| Phase Interaction | Gas stripping out volatile solutes from liquid | Gas absorption into liquid solvent |
| Operation Mode | Gas flows upwards; liquid flows downwards | Gas flows upwards; liquid flows downwards |
| Typical Applications | Removal of CO2, ammonia, or other volatiles from water or solvents | Removal of SO2, H2S, or pollutants from gas streams |
| Design Focus | Maximize mass transfer of solutes from liquid to gas | Maximize gas-liquid contact to dissolve gases in liquid |
| Thermodynamics | Driven by volatility difference and gas partial pressure | Driven by solubility and chemical affinity in solvent |
| Common Packing | Random or structured packing to enhance gas-liquid contact | Random or structured packing to increase absorption efficiency |
Introduction to Stripping and Absorption Columns
Stripping columns remove volatile components from a liquid by transferring them into a gas phase, relying on differences in volatility and mass transfer principles. Absorption columns capture specific gas components into a liquid solvent, utilizing solubility and chemical affinity to separate target compounds. Both columns operate based on mass transfer efficiency but differ primarily in the direction of component transfer and their industrial applications.
Fundamental Principles of Stripping Columns
Stripping columns operate on the principle of removing volatile components from a liquid mixture by transferring them into a vapor phase, driven by a concentration gradient and temperature difference. Unlike absorption columns, which capture gases into a liquid, stripping relies on heating the liquid feed to facilitate mass transfer from liquid to vapor. Your process efficiency depends heavily on the vapor-liquid equilibrium and proper tray or packing design to maximize contact area and separation performance.
Core Concepts of Absorption Columns
Absorption columns utilize a liquid solvent to selectively remove specific components from a gas mixture, relying on solubility differences and mass transfer principles to achieve separation. Core concepts include the equilibrium between gas and liquid phases, mass transfer efficiency, and stage-wise contact to optimize solvent-gas interaction. Your focus on absorption columns highlights their role in purifying gases by capturing target pollutants or valuable compounds through controlled absorption processes.
Key Differences Between Stripping and Absorption Columns
Stripping columns and absorption columns differ primarily in their operational objectives; stripping columns remove volatile components from a liquid by transferring them to a gas phase, whereas absorption columns capture specific gases from a gas stream into a liquid solvent. The design parameters also contrast, with stripping columns optimized for vapor-liquid contact using steam or inert gases, while absorption columns employ selective solvents for targeted gas absorption. Material selection and process conditions such as temperature and pressure vary accordingly to maximize mass transfer efficiency in each type of column.
Design Considerations for Stripping Columns
Design considerations for stripping columns center on maximizing mass transfer efficiency by selecting appropriate packing materials or trays to promote contact between liquid and vapor phases. Controlling operating parameters such as temperature, pressure, and flow rates ensures optimal removal of volatile components from the liquid feed. Your design must also address column height and diameter to balance capacity with pressure drop, while considering factors like feed composition and desired purity.
Design Parameters for Absorption Columns
Design parameters for absorption columns include gas and liquid flow rates, column diameter, packing type and height, and mass transfer coefficients that influence the efficiency of solute removal. Liquid-to-gas ratio and residence time are critical for maximizing solute absorption while minimizing energy consumption. Column internals such as trays or packings are chosen based on pressure drop considerations and desired mass transfer surface area to optimize the absorption process.
Common Applications of Stripping Columns
Stripping columns are widely used in wastewater treatment to remove volatile contaminants such as ammonia and hydrogen sulfide from water streams. Unlike absorption columns which capture gases into a liquid phase, stripping columns focus on transferring volatile compounds from a liquid feed into a gas phase for further processing or disposal. You will commonly find stripping columns in industries like petroleum refining, chemical manufacturing, and environmental engineering where efficient gas-liquid separation is critical.
Industrial Uses of Absorption Columns
Absorption columns are widely used in industries for gas purification, such as removing sulfur dioxide from flue gases in power plants and capturing carbon dioxide in natural gas processing. These columns facilitate the transfer of specific components from a gas phase into a liquid solvent, enhancing product quality and environmental compliance. Stripping columns, by contrast, are primarily employed to remove volatile compounds from liquid mixtures, focusing on the recovery or purification of solvents rather than gas absorption.
Operational Challenges and Troubleshooting
Stripping columns commonly face challenges such as flooding, foaming, and channeling, which can cause inefficiencies in mass transfer and increased pressure drop, while absorption columns often struggle with solvent degradation and corrosion due to the nature of the absorbent. Troubleshooting stripping columns typically involves adjusting reflux ratios and reboiler duties to maintain optimal vapor-liquid contact and prevent flooding, whereas absorption columns require frequent monitoring of solvent quality and temperature control to avoid loss of absorption capacity. Both processes benefit from regular inspection of packing or trays to mitigate maldistribution and ensure effective mass transfer.
Choosing Between Stripping and Absorption Columns
Choosing between stripping and absorption columns depends on your process goals: stripping columns remove volatile components from a liquid by transferring them into a gas phase, while absorption columns capture specific gases from a gas stream into a liquid solvent. Key factors influencing the decision include the volatility of components, desired separation efficiency, and solvent compatibility. Optimizing mass transfer area, packing type, and operating conditions enhances performance tailored to your separation needs.
Stripping column vs absorption column Infographic
 libmatt.com
libmatt.com