The Wurtz reaction involves the coupling of two alkyl halides using sodium metal to form a higher alkane, primarily focusing on simple alkyl chains. Your choice between the Wurtz and Fittig reactions depends on the desired product, as the Fittig reaction couples aryl halides instead, producing biaryl compounds through sodium-mediated coupling.
Table of Comparison
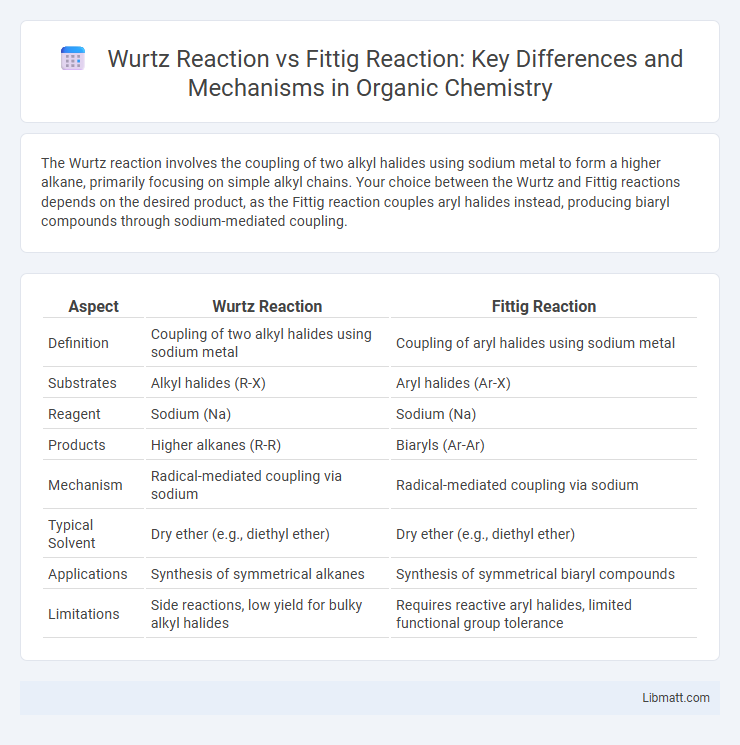
| Aspect | Wurtz Reaction | Fittig Reaction |
|---|---|---|
| Definition | Coupling of two alkyl halides using sodium metal | Coupling of aryl halides using sodium metal |
| Substrates | Alkyl halides (R-X) | Aryl halides (Ar-X) |
| Reagent | Sodium (Na) | Sodium (Na) |
| Products | Higher alkanes (R-R) | Biaryls (Ar-Ar) |
| Mechanism | Radical-mediated coupling via sodium | Radical-mediated coupling via sodium |
| Typical Solvent | Dry ether (e.g., diethyl ether) | Dry ether (e.g., diethyl ether) |
| Applications | Synthesis of symmetrical alkanes | Synthesis of symmetrical biaryl compounds |
| Limitations | Side reactions, low yield for bulky alkyl halides | Requires reactive aryl halides, limited functional group tolerance |
Introduction to Wurtz and Fittig Reactions
Wurtz reaction involves the coupling of two alkyl halides using sodium metal in dry ether to form higher alkanes, primarily used for symmetric alkane synthesis. Fittig reaction is a similar coupling process but specifically uses aryl halides, producing symmetrical biaryl compounds through reaction with sodium metal. Both reactions are fundamental in organic synthesis for forming carbon-carbon bonds, with Wurtz suited for alkyl chains and Fittig for aromatic systems.
Historical Background
The Wurtz reaction, developed by Charles Adolphe Wurtz in 1855, marked a significant advancement in organic synthesis by enabling the coupling of alkyl halides through sodium metal to form higher alkanes. Shortly after, Rudolf Fittig expanded this methodology in 1860 with the Fittig reaction, which involved the coupling of aryl halides under similar conditions to produce biaryl compounds. Your understanding of these reactions' historical context highlights their foundational role in the development of organometallic chemistry and synthetic strategies.
Reaction Mechanisms
The Wurtz reaction involves the coupling of two alkyl halides in the presence of sodium metal to form a new carbon-carbon bond through a radical mechanism starting with single electron transfer. In contrast, the Fittig reaction couples aryl halides with sodium metal via a similar single electron transfer process but typically involves aromatic substrates leading to biaryl compounds. Both reactions proceed through radical intermediates, but their substrate specificity and product types differentiate their underlying mechanisms.
Key Reagents and Conditions
The Wurtz reaction primarily involves the coupling of alkyl halides using sodium metal in dry ether as the key reagent and solvent under anhydrous conditions. In contrast, the Fittig reaction uses aryl halides with sodium metal, often requiring elevated temperatures and strictly anhydrous ether solvents to facilitate the formation of biaryl compounds. Your choice between these reactions depends on whether you aim to couple alkyl or aryl groups, as the reagents and reaction conditions differ accordingly.
Types of Compounds Formed
The Wurtz reaction primarily forms symmetrical alkanes through the coupling of alkyl halides using sodium metal, producing compounds such as ethane or butane. The Fittig reaction, on the other hand, couples aryl halides to create biaryl compounds, which are important in the synthesis of complex aromatic structures. Your choice between these reactions depends on whether you need saturated hydrocarbons or aromatic biaryl compounds.
Comparative Analysis: Wurtz vs Fittig
The Wurtz reaction involves the coupling of two identical alkyl halides using sodium metal to form symmetric alkanes, whereas the Fittig reaction couples aryl halides, often producing biaryl compounds through similar sodium-mediated reductive coupling. Wurtz reaction is typically limited to simpler alkyl halides due to side reactions and low yield with bulky or functionalized substrates, while the Fittig reaction is more suited for aryl or aryl-alkyl halides, offering higher specificity in forming carbon-carbon bonds between aromatic rings. Both reactions rely on sodium metal for electron transfer but differ in substrate scope, reaction conditions, and product types, making each preferable depending on targeted synthesis goals in organic chemistry.
Applications in Organic Synthesis
The Wurtz reaction is primarily used for the synthesis of symmetrical and some unsymmetrical alkanes by coupling alkyl halides with sodium metal, making it valuable in forming carbon-carbon bonds in simple hydrocarbon chains. The Fittig reaction, a variation of the Wurtz reaction, specifically couples aryl halides to form biaryl compounds, which are important intermediates in pharmaceuticals, agrochemicals, and polymer industries. Your choice between these reactions depends on whether the target molecule is an alkane or a biaryl, influencing the design of synthetic pathways in organic chemistry.
Limitations and Challenges
The Wurtz reaction faces limitations such as poor tolerance to functional groups and difficulty coupling sterically hindered alkyl halides, often leading to low yields and side reactions like elimination or rearrangement. The Fittig reaction shares similar challenges but is restricted to aryl halides, where competing side reactions like dehalogenation reduce efficiency and limit substrate scope. Your choice between these reactions should consider these constraints to optimize product yield and purity in synthesis planning.
Recent Advances and Modifications
Recent advances in the Wurtz reaction involve the use of milder and more selective reagents, such as microwave-assisted synthesis and solid-supported alkali metals, to improve reaction efficiency and reduce side-product formation. Modifications in the Fittig reaction include employing transition-metal catalysts and tailored ligands to enhance coupling yields and broaden substrate scope for the synthesis of biaryl compounds. Both reactions benefit from green chemistry approaches, like solvent-free conditions and recyclable catalysts, promoting sustainability in carbon-carbon bond formation.
Summary Table: Wurtz vs Fittig Reaction
The Wurtz reaction involves the coupling of two identical alkyl halides using sodium metal to form symmetric alkanes, while the Fittig reaction couples aryl halides under similar conditions to yield biaryl compounds. Both reactions utilize sodium as a reductant, but the Wurtz reaction is primarily applied to alkyl halides whereas the Fittig reaction targets aromatic halides. Reaction conditions often require dry ether solvents to stabilize intermediates and promote efficient coupling in both methodologies.
Wurtz reaction vs Fittig reaction Infographic
 libmatt.com
libmatt.com