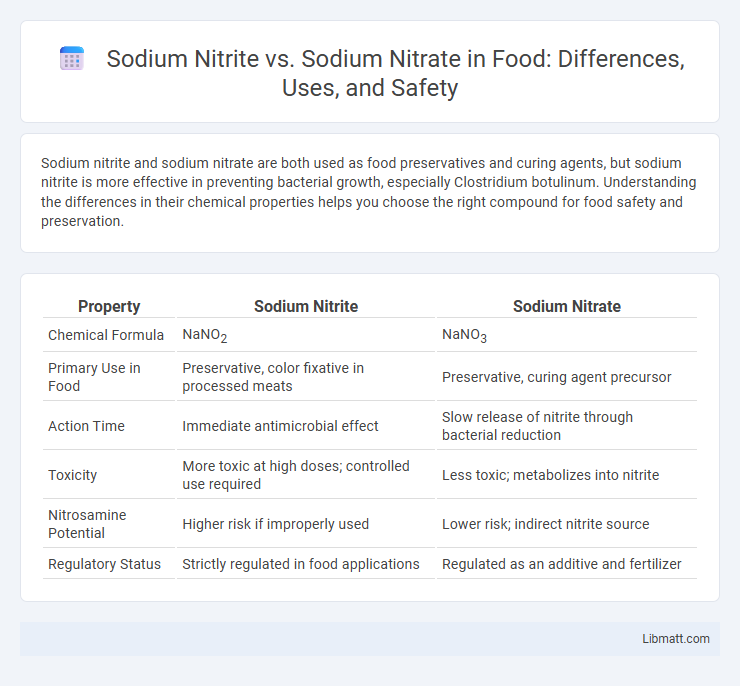
Sodium nitrite and sodium nitrate are both used as food preservatives and curing agents, but sodium nitrite is more effective in preventing bacterial growth, especially Clostridium botulinum. Understanding the differences in their chemical properties helps you choose the right compound for food safety and preservation.
Table of Comparison
| Property | Sodium Nitrite | Sodium Nitrate |
|---|---|---|
| Chemical Formula | NaNO2 | NaNO3 |
| Primary Use in Food | Preservative, color fixative in processed meats | Preservative, curing agent precursor |
| Action Time | Immediate antimicrobial effect | Slow release of nitrite through bacterial reduction |
| Toxicity | More toxic at high doses; controlled use required | Less toxic; metabolizes into nitrite |
| Nitrosamine Potential | Higher risk if improperly used | Lower risk; indirect nitrite source |
| Regulatory Status | Strictly regulated in food applications | Regulated as an additive and fertilizer |
Introduction to Sodium Nitrite and Sodium Nitrate
Sodium nitrite (NaNO2) and sodium nitrate (NaNO3) are inorganic compounds widely used as food preservatives and industrial chemicals. Sodium nitrite serves primarily as a curing agent in processed meats to inhibit bacterial growth and enhance color, while sodium nitrate acts as a fertilizer and a precursor to sodium nitrite through bacterial reduction. Both compounds contain nitrogen and oxygen atoms but differ in oxidation states and chemical properties, influencing their applications and safety considerations.
Chemical Structure and Composition Differences
Sodium nitrite (NaNO2) contains one nitrogen atom bonded to two oxygen atoms, with a nitrogen in a +3 oxidation state, while sodium nitrate (NaNO3) has one nitrogen atom bonded to three oxygen atoms, with nitrogen in a +5 oxidation state. This structural difference results in sodium nitrite having a bent molecular geometry and sodium nitrate a trigonal planar shape, influencing their chemical reactivity and stability. Understanding these distinctions helps you choose the appropriate compound for applications like food preservation or industrial processes.
Natural Occurrence and Sources
Sodium nitrite naturally occurs in certain vegetables like spinach and celery, where it forms through bacterial action on nitrates. Sodium nitrate is commonly found in soil and mineral deposits, especially in arid regions such as the Atacama Desert in Chile. Both compounds are also present in cured meats as preservatives, with sodium nitrate often converting to sodium nitrite during processing.
Industrial and Food Applications
Sodium nitrite is widely used in the food industry as a curing agent for meats, preventing bacterial growth and enhancing color, while sodium nitrate serves both as a preservative and a fertilizer in agriculture due to its high nitrogen content. Industrially, sodium nitrite is essential in the manufacture of dyes, pharmaceuticals, and corrosion inhibitors, whereas sodium nitrate is primarily utilized in explosives, glass production, and as a heat transfer salt in solar power plants. Both compounds play critical roles in food safety and industrial processes, with their unique chemical properties dictating specific applications.
Role in Food Preservation and Safety
Sodium nitrite plays a critical role in food preservation by inhibiting the growth of Clostridium botulinum, the bacteria responsible for botulism, thereby ensuring meat products like cured meats and sausages are safe for consumption. Sodium nitrate serves as a precursor that gradually converts to sodium nitrite through bacterial action during the curing process, extending the preservation effect over time. Both compounds contribute to the characteristic color and flavor of cured meats while enhancing shelf life and preventing spoilage.
Health Implications and Potential Risks
Sodium nitrite and sodium nitrate both have health implications due to their role in forming nitrosamines, which are carcinogenic compounds linked to increased cancer risk, particularly colorectal cancer. While sodium nitrite is more reactive and directly associated with methemoglobinemia, a condition that impairs oxygen transport in blood, sodium nitrate primarily poses risks through its conversion to nitrite in the body. Excessive consumption of processed meats containing these additives correlates with negative health outcomes, emphasizing the need for regulated intake and monitoring in food products.
Regulatory Guidelines and Safety Limits
Sodium nitrite and sodium nitrate are regulated differently due to their distinct chemical properties and uses, with sodium nitrite facing stricter safety limits in food preservation to prevent toxic effects such as methemoglobinemia. Regulatory agencies like the FDA and EFSA set maximum allowable concentrations for these compounds in meat products, with sodium nitrite typically limited to 120 ppm whereas sodium nitrate limits vary but are generally higher due to lower immediate toxicity. Your compliance with these guidelines is crucial to ensure safe consumption and avoid health risks associated with excessive intake of nitrites and nitrates.
Environmental Impact and Handling
Sodium nitrite and sodium nitrate have distinct environmental impacts due to their differing chemical properties and applications in agriculture and industry. Sodium nitrate, commonly used as a fertilizer, can contribute to groundwater contamination and eutrophication if over-applied, posing risks to aquatic ecosystems. Sodium nitrite requires careful handling due to its toxicity and potential to form harmful nitrosamines, making safe storage and usage crucial to protect your health and minimize environmental hazards.
How to Distinguish Between Sodium Nitrite and Sodium Nitrate
Sodium nitrite (NaNO2) and sodium nitrate (NaNO3) can be distinguished by their chemical composition and physical properties; sodium nitrite contains two oxygen atoms and is a pale yellow crystalline powder, while sodium nitrate contains three oxygen atoms and appears as white crystals. Testing their solubility in water reveals sodium nitrate dissolves more readily, and their reactions with acid produce distinct gas emissions--nitrous acid for sodium nitrite versus nitric acid derivatives for sodium nitrate. Your identification process can include simple chemical tests such as the brown ring test, which identifies nitrate ions uniquely, ensuring accurate differentiation between the two compounds.
Summary: Key Differences and Practical Considerations
Sodium nitrite (NaNO2) and sodium nitrate (NaNO3) differ primarily in their chemical composition and applications; sodium nitrite contains one less oxygen atom, making it a stronger oxidizing agent widely used as a preservative and color fixative in cured meats. Sodium nitrate, with an extra oxygen atom, serves mainly as a fertilizer and a precursor in industrial processes, exhibiting slower reactivity compared to sodium nitrite. Practical considerations include the toxicity levels and regulatory limits, where sodium nitrite requires careful dosage control due to its potential to form carcinogenic nitrosamines under certain conditions.
Sodium Nitrite vs Sodium Nitrate Infographic
 libmatt.com
libmatt.com