Electroplating enhances your metal surfaces by depositing a thin layer of metal to improve corrosion resistance and aesthetic appeal, while anodizing creates a durable oxide layer on aluminum to increase hardness and wear resistance. Both processes serve to protect and enhance metals but differ in technique, materials used, and the types of finishes produced.
Table of Comparison
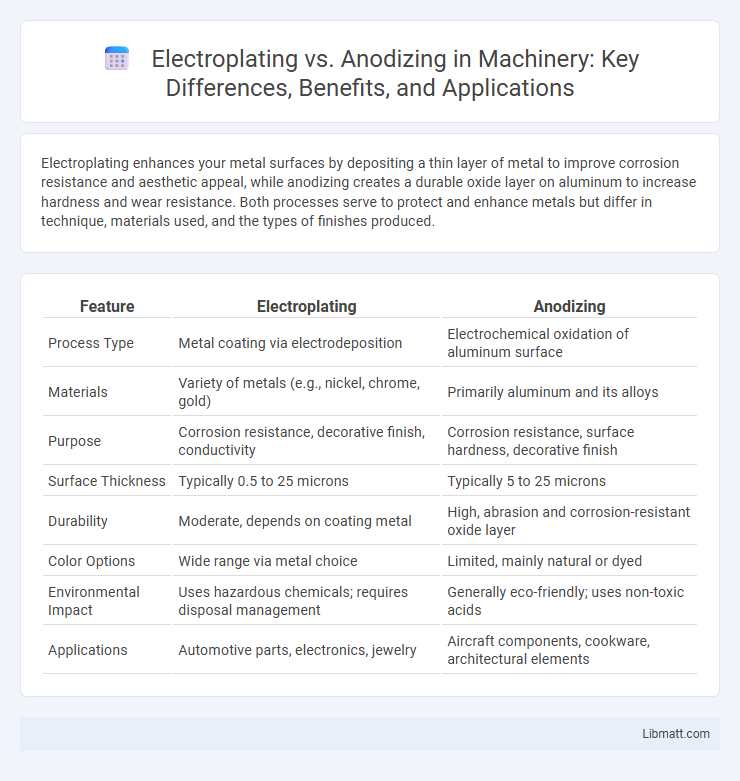
| Feature | Electroplating | Anodizing |
|---|---|---|
| Process Type | Metal coating via electrodeposition | Electrochemical oxidation of aluminum surface |
| Materials | Variety of metals (e.g., nickel, chrome, gold) | Primarily aluminum and its alloys |
| Purpose | Corrosion resistance, decorative finish, conductivity | Corrosion resistance, surface hardness, decorative finish |
| Surface Thickness | Typically 0.5 to 25 microns | Typically 5 to 25 microns |
| Durability | Moderate, depends on coating metal | High, abrasion and corrosion-resistant oxide layer |
| Color Options | Wide range via metal choice | Limited, mainly natural or dyed |
| Environmental Impact | Uses hazardous chemicals; requires disposal management | Generally eco-friendly; uses non-toxic acids |
| Applications | Automotive parts, electronics, jewelry | Aircraft components, cookware, architectural elements |
Introduction to Electroplating and Anodizing
Electroplating involves depositing a thin layer of metal onto a surface using an electric current, enhancing corrosion resistance, appearance, and conductivity. Anodizing is an electrochemical process that thickens the natural oxide layer on metals like aluminum to improve durability, corrosion resistance, and color options. Understanding these surface treatments helps you choose the right method for protecting and finishing metal products.
Fundamental Principles of Electroplating
Electroplating involves using an electric current to reduce dissolved metal ions, forming a coherent metal coating on a conductive surface, enhancing corrosion resistance and aesthetic appeal. This process requires an electrolyte solution containing the desired metal ions, a cathode (the part to be plated), and an anode made of the plating metal or inert material. Understanding this principle allows you to choose electroplating for applications demanding precise metal layering and improved surface properties.
Key Processes in Anodizing
Anodizing involves an electrochemical process where your metal is immersed in an acid electrolyte bath and an electric current is passed through to create a thick oxide layer on the surface, enhancing corrosion resistance and durability. This oxide layer is porous initially, allowing for dye absorption or sealing for additional protection and aesthetic customization. Unlike electroplating, anodizing modifies the surface rather than depositing a separate metal layer, making it ideal for aluminum and other lightweight metals.
Material Compatibility: What Can Be Treated?
Electroplating is suitable for a wide range of metals including steel, copper, and aluminum, providing a durable metal coating that enhances corrosion resistance and conductivity. Anodizing specifically treats aluminum and its alloys, creating a thick oxide layer that improves surface hardness and corrosion resistance without adding metal layers. Understanding your material compatibility ensures the chosen process optimally protects and enhances your parts.
Surface Finish and Appearance Differences
Electroplating provides a smooth, shiny surface finish by depositing a metal coating such as nickel or chromium, enhancing corrosion resistance and aesthetic appeal. Anodizing creates a durable, matte to glossy finish through an electrochemical process that thickens the natural oxide layer on aluminum, allowing for vibrant color options and improved surface hardness. Your choice depends on whether you prioritize a metallic luster from electroplating or the durable, colorful finish offered by anodizing.
Performance: Durability and Corrosion Resistance
Electroplating provides a thin metal coating that enhances surface hardness and offers moderate corrosion resistance, making it suitable for decorative and protective applications. Anodizing transforms the metal surface into a thick oxide layer, significantly improving durability and providing exceptional corrosion resistance, especially on aluminum parts. Your choice between electroplating and anodizing depends on the desired balance of aesthetic appeal, wear resistance, and environmental protection.
Industrial and Common Applications
Electroplating is widely used in automotive parts, electronics, and jewelry to enhance corrosion resistance, wear protection, and aesthetic appeal by depositing a metal layer such as nickel, chromium, or gold. Anodizing is predominantly applied to aluminum components in aerospace, construction, and consumer goods, improving surface durability, corrosion resistance, and providing a porous oxide layer for better paint adhesion. Both processes serve critical roles in manufacturing, with electroplating favored for conductive coatings and anodizing for creating robust, non-metallic oxide surfaces.
Environmental and Safety Considerations
Electroplating often involves toxic chemicals like cyanide and heavy metals, which pose significant environmental hazards if not properly managed, requiring strict waste disposal protocols to prevent soil and water contamination. Anodizing, on the other hand, primarily uses sulfuric acid and produces less hazardous waste, making it generally safer and more environmentally friendly, though handling acids still demands careful safety measures to protect workers. Both processes require adherence to regulatory standards such as OSHA and EPA guidelines to minimize health risks and environmental impact.
Cost Comparison: Electroplating vs Anodizing
Electroplating generally incurs higher material and labor costs due to the use of expensive metals like nickel or chrome and complex plating processes, whereas anodizing is more cost-effective, especially for aluminum parts, thanks to its simpler chemical treatment and lower material expenses. Electroplating requires precise control and waste management, increasing overall operational costs, while anodizing involves less hazardous waste and reduced environmental compliance costs. For large-scale production, anodizing offers better cost efficiency, but electroplating is preferred when enhanced surface properties are necessary despite the higher price.
Choosing the Right Method for Your Needs
Electroplating provides a metallic coating that enhances corrosion resistance and aesthetic appeal, ideal for decorative or conductive applications. Anodizing creates a durable oxide layer that improves surface hardness and wear resistance, making it suitable for aluminum parts exposed to harsh environments. Understanding your material type, desired finish, and environmental exposure helps determine whether electroplating or anodizing best meets your needs.
Electroplating vs anodizing Infographic
 libmatt.com
libmatt.com