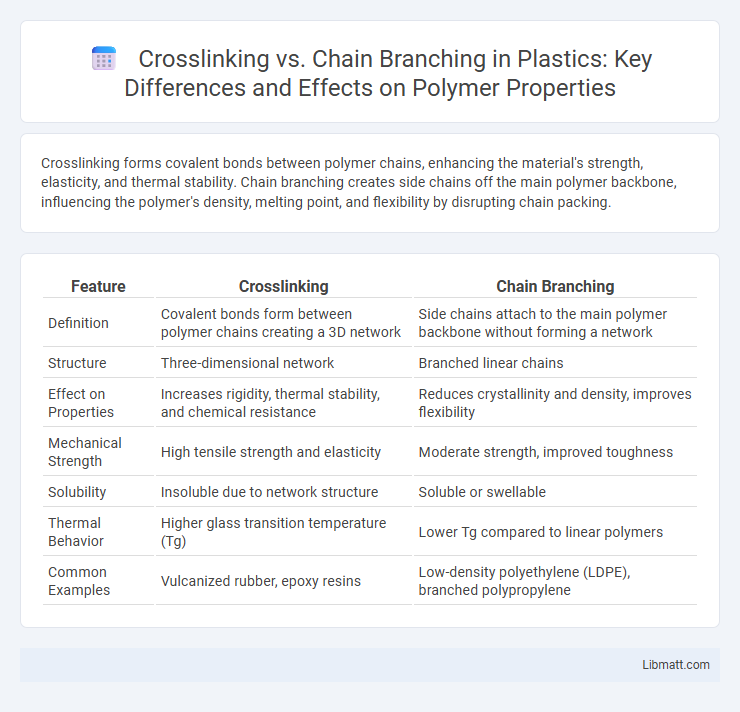
Crosslinking forms covalent bonds between polymer chains, enhancing the material's strength, elasticity, and thermal stability. Chain branching creates side chains off the main polymer backbone, influencing the polymer's density, melting point, and flexibility by disrupting chain packing.
Table of Comparison
| Feature | Crosslinking | Chain Branching |
|---|---|---|
| Definition | Covalent bonds form between polymer chains creating a 3D network | Side chains attach to the main polymer backbone without forming a network |
| Structure | Three-dimensional network | Branched linear chains |
| Effect on Properties | Increases rigidity, thermal stability, and chemical resistance | Reduces crystallinity and density, improves flexibility |
| Mechanical Strength | High tensile strength and elasticity | Moderate strength, improved toughness |
| Solubility | Insoluble due to network structure | Soluble or swellable |
| Thermal Behavior | Higher glass transition temperature (Tg) | Lower Tg compared to linear polymers |
| Common Examples | Vulcanized rubber, epoxy resins | Low-density polyethylene (LDPE), branched polypropylene |
Introduction to Polymer Crosslinking and Chain Branching
Polymer crosslinking involves forming covalent bonds between polymer chains, creating a three-dimensional network that enhances mechanical strength, thermal stability, and chemical resistance. Chain branching refers to the attachment of side chains onto the main polymer backbone, influencing viscosity, density, and melting properties without creating a network structure. Understanding the differences between crosslinking and chain branching is crucial for tailoring polymer properties for applications in adhesives, elastomers, and thermosetting plastics.
Fundamental Definitions: Crosslinking vs Chain Branching
Crosslinking refers to the formation of covalent bonds between polymer chains, creating a three-dimensional network that enhances material strength, thermal stability, and chemical resistance. Chain branching involves the attachment of side chains to the main polymer backbone without forming inter-chain bonds, affecting polymer density, crystallinity, and melt viscosity. The fundamental difference lies in crosslinking producing interconnected networks, whereas chain branching alters the polymer's molecular architecture without linking separate chains.
Chemical Mechanisms Behind Crosslinking
Crosslinking involves the formation of covalent or ionic bonds between polymer chains, creating a three-dimensional network that enhances mechanical strength and thermal stability. The chemical mechanism typically includes free radical initiation, where reactive species generate covalent bridges, linking polymer segments. Chain branching, in contrast, introduces side chains without forming extensive interchain bonds, altering polymer properties by increasing molecular weight and viscosity without establishing a crosslinked network.
Chain Branching: Types and Formation Processes
Chain branching occurs when side chains form along the main polymer backbone, creating different branch types such as short-chain and long-chain branches. Short-chain branching typically arises during polymerization through chain transfer reactions, while long-chain branching results from branching agents or specific monomer sequences. These branching variations influence polymer properties like density, melt flow, and mechanical strength, distinguishing chain branching processes from crosslinking.
Impact on Polymer Physical Properties
Crosslinking forms covalent bonds between polymer chains, significantly enhancing mechanical strength, thermal stability, and resistance to solvents by creating a three-dimensional network. Chain branching introduces side chains that improve polymer processability and impact flexibility while reducing crystallinity and tensile strength. The degree and type of crosslinking or branching directly influence polymer density, elasticity, and glass transition temperature, determining its suitability for applications such as elastomers or thermoplastics.
Thermal and Mechanical Stability Differences
Crosslinking forms covalent bonds between polymer chains, significantly enhancing thermal stability by creating a network that resists flow and deformation at high temperatures. Chain branching introduces side chains that increase molecular entanglement, improving mechanical strength and elasticity but offering less thermal resistance compared to crosslinked structures. As a result, crosslinked polymers exhibit superior dimensional stability and mechanical durability under thermal stress, while branched polymers provide better flexibility and impact resistance.
Applications in Polymer Industry
Crosslinking enhances polymer properties such as thermal stability, chemical resistance, and mechanical strength, making it ideal for applications in adhesives, coatings, and rubber vulcanization. Chain branching influences polymer density and crystallinity, affecting materials used in packaging films and plastics like low-density polyethylene (LDPE). Understanding these mechanisms allows you to tailor polymer characteristics for specific industrial applications, optimizing performance and durability.
Advantages and Limitations of Crosslinking
Crosslinking enhances polymer strength, thermal stability, and chemical resistance by forming covalent bonds between polymer chains, creating a three-dimensional network. Its primary limitation is reduced processability and elasticity due to restricted chain mobility, which can lead to brittleness in highly crosslinked materials. Crosslinked polymers are also difficult to recycle or reprocess, posing environmental challenges compared to non-crosslinked, branched polymers.
Chain Branching: Benefits and Drawbacks
Chain branching in polymers enhances material flexibility and impact resistance by introducing side chains that disrupt tight packing, leading to improved toughness and processability. However, excessive branching can reduce crystallinity, which lowers thermal stability and mechanical strength. Your choice between crosslinking and chain branching depends on the desired balance of elasticity, durability, and heat resistance for specific applications.
Choosing the Right Modification: Crosslinking or Chain Branching?
Selecting between crosslinking and chain branching depends on desired polymer properties: crosslinking creates a three-dimensional network, enhancing rigidity, thermal stability, and chemical resistance, suitable for applications needing durability. Chain branching increases polymer chain side groups, improving flexibility, impact resistance, and processability, ideal for materials requiring elasticity and toughness. Evaluating end-use requirements, mechanical performance, and environmental exposure guides the optimal modification choice.
Crosslinking vs chain branching Infographic
 libmatt.com
libmatt.com