ISO 9001 focuses on general quality management systems applicable across various industries, ensuring consistent product quality and customer satisfaction, while ISO 13485 is specifically tailored for the medical device industry, emphasizing regulatory compliance and risk management in the design and manufacture of plastic components. Understanding the differences helps you implement the most relevant standards for quality assurance in plastic production processes.
Table of Comparison
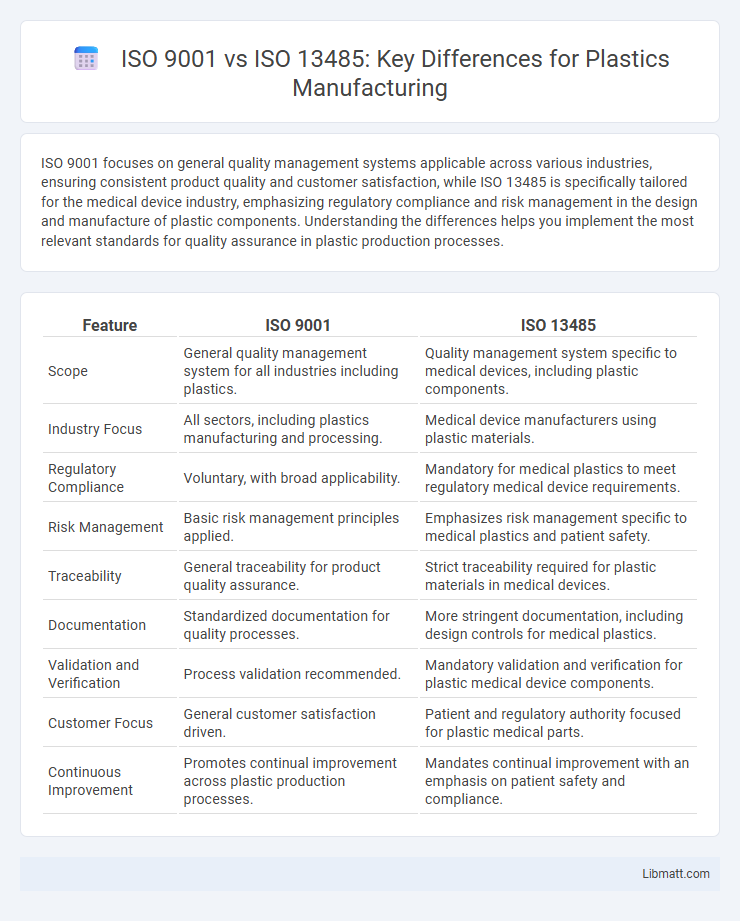
| Feature | ISO 9001 | ISO 13485 |
|---|---|---|
| Scope | General quality management system for all industries including plastics. | Quality management system specific to medical devices, including plastic components. |
| Industry Focus | All sectors, including plastics manufacturing and processing. | Medical device manufacturers using plastic materials. |
| Regulatory Compliance | Voluntary, with broad applicability. | Mandatory for medical plastics to meet regulatory medical device requirements. |
| Risk Management | Basic risk management principles applied. | Emphasizes risk management specific to medical plastics and patient safety. |
| Traceability | General traceability for product quality assurance. | Strict traceability required for plastic materials in medical devices. |
| Documentation | Standardized documentation for quality processes. | More stringent documentation, including design controls for medical plastics. |
| Validation and Verification | Process validation recommended. | Mandatory validation and verification for plastic medical device components. |
| Customer Focus | General customer satisfaction driven. | Patient and regulatory authority focused for plastic medical parts. |
| Continuous Improvement | Promotes continual improvement across plastic production processes. | Mandates continual improvement with an emphasis on patient safety and compliance. |
Introduction to ISO 9001 and ISO 13485 Standards
ISO 9001 is a globally recognized quality management standard applicable across various industries, emphasizing process consistency and customer satisfaction. ISO 13485 specifically targets the medical device sector, focusing on regulatory compliance and risk management within the design and manufacturing of medical-grade plastics. Your choice between these standards depends on whether your plastics are intended for general manufacturing or regulated medical environments.
Key Differences Between ISO 9001 and ISO 13485
ISO 9001 is a general quality management standard applicable to any industry, emphasizing customer satisfaction, continuous improvement, and process efficiency. ISO 13485 specifically addresses quality management systems for medical devices, including plastics used in medical applications, with stringent regulatory compliance, risk management, and product traceability requirements. The key differences lie in ISO 13485's focus on regulatory controls, documentation rigor, and mandatory process validation tailored to medical-grade plastic manufacturing.
Importance of Quality Management in Plastics Manufacturing
ISO 9001 and ISO 13485 both emphasize quality management but serve different purposes in plastics manufacturing, with ISO 9001 focusing on general quality management systems and ISO 13485 targeting medical device-related plastics production. Implementing ISO 9001 ensures consistent product quality and operational efficiency, while ISO 13485 addresses stringent regulatory requirements essential for plastics used in medical applications. Effective quality management in plastics manufacturing reduces defects, enhances customer satisfaction, and ensures compliance with industry standards critical to product safety and performance.
Regulatory Requirements for Plastics in Medical Devices
ISO 13485 specifically addresses regulatory requirements for plastics used in medical devices, ensuring compliance with strict quality management systems tailored to medical applications. ISO 9001 provides a general framework for quality management but lacks the detailed regulatory focus necessary for medical-grade plastics. Manufacturers of medical devices rely on ISO 13485 certification to meet global medical regulatory standards for biocompatibility, traceability, and risk management of plastic components.
Scope and Applicability of ISO 9001 vs ISO 13485
ISO 9001 applies broadly to quality management systems across various industries, emphasizing customer satisfaction and continual improvement in processes related to product manufacturing, including plastics. In contrast, ISO 13485 is specifically designed for medical device manufacturers, ensuring regulatory compliance and risk management in the production of plastic components used in medical applications. The scope of ISO 13485 is narrower but more stringent, prioritizing safety and effectiveness of medical-grade plastics throughout the entire lifecycle.
Documentation and Record-Keeping Differences
ISO 9001 in plastics emphasizes general quality management system documentation and record-keeping to ensure consistent product quality, while ISO 13485 requires more stringent documentation tailored to medical devices, including detailed traceability and regulatory compliance records. Your plastics manufacturing process under ISO 13485 demands comprehensive control of design, production, and validation documents to meet medical device standards that surpass the broader quality focus of ISO 9001. The difference lies in ISO 13485's emphasis on maintaining thorough, controlled, and auditable records critical for patient safety and regulatory approval.
Risk Management Approaches in Plastic Manufacturing
ISO 9001 emphasizes a broad risk-based thinking approach applicable to various industries, including plastics manufacturing, fostering continuous improvement and customer satisfaction. ISO 13485, tailored specifically for medical device plastics, mandates rigorous risk management processes aligned with regulatory requirements to ensure product safety and efficacy. Your plastic manufacturing operations benefit from integrating ISO 13485's detailed risk control procedures when producing medical-grade plastics, enhancing quality and compliance.
Training and Competency Requirements
ISO 9001 emphasizes general training and competency requirements to ensure quality management across various industries, including plastics. ISO 13485, tailored for medical device manufacturing within plastics, enforces more stringent training protocols focused on regulatory compliance, risk management, and product safety. Your organization must align training programs with the specific standard relevant to your product's end use for optimal compliance and quality assurance.
Audit and Certification Processes for Plastics Companies
ISO 9001 and ISO 13485 certification processes for plastics companies differ significantly in audit scope and regulatory requirements. While ISO 9001 audits primarily assess quality management systems applicable across industries, ISO 13485 audits focus intensively on compliance with medical device regulations, including strict documentation and risk management specific to plastic components used in healthcare. Your plastics company must prepare for more rigorous and frequent ISO 13485 assessments to meet medical sector standards, whereas ISO 9001 certification involves broader, less specialized audit criteria.
Choosing the Right Standard for Your Plastics Business
ISO 9001 and ISO 13485 serve distinct purposes for plastics businesses, with ISO 9001 focusing on general quality management systems applicable across industries, while ISO 13485 targets medical device manufacturing, emphasizing stringent regulatory compliance and risk management. Choosing ISO 9001 enhances your overall operational efficiency and customer satisfaction through a flexible framework, whereas ISO 13485 is essential if your plastics products are used in medical applications requiring strict adherence to regulatory standards. Understanding your market demands and regulatory environment ensures you select the standard that best supports your business objectives and product quality.
ISO 9001 vs ISO 13485 in Plastics Infographic
 libmatt.com
libmatt.com