Styrene is a liquid hydrocarbon monomer used as the building block for polystyrene, a solid, durable plastic polymer commonly used in packaging, insulation, and disposable containers. Understanding the difference between styrene as a raw chemical and polystyrene as a finished material can help you make informed decisions in manufacturing or product design.
Table of Comparison
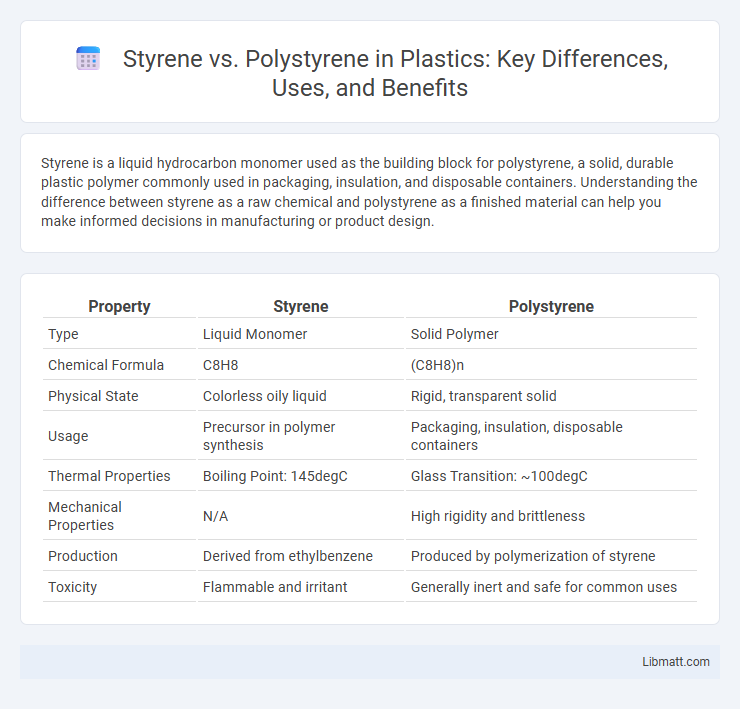
| Property | Styrene | Polystyrene |
|---|---|---|
| Type | Liquid Monomer | Solid Polymer |
| Chemical Formula | C8H8 | (C8H8)n |
| Physical State | Colorless oily liquid | Rigid, transparent solid |
| Usage | Precursor in polymer synthesis | Packaging, insulation, disposable containers |
| Thermal Properties | Boiling Point: 145degC | Glass Transition: ~100degC |
| Mechanical Properties | N/A | High rigidity and brittleness |
| Production | Derived from ethylbenzene | Produced by polymerization of styrene |
| Toxicity | Flammable and irritant | Generally inert and safe for common uses |
Introduction to Styrene and Polystyrene
Styrene is a colorless, oily liquid used as a monomer to produce polystyrene, a versatile synthetic polymer widely utilized in packaging, insulation, and consumer products. Polystyrene forms through the polymerization of styrene molecules, resulting in a lightweight, rigid material with excellent insulating properties and ease of molding. The chemical structure of styrene features a vinyl group attached to a benzene ring, which imparts distinct reactivity leading to the formation of polystyrene's durable polymer chains.
Chemical Structure: Styrene vs Polystyrene
Styrene is a monomer with the chemical formula C8H8, featuring a vinyl group attached to a benzene ring, which enables polymerization through its double bond. Polystyrene is a polymer consisting of repeating styrene units linked via covalent bonds in a long chain, resulting in a solid thermoplastic material. The transformation from styrene to polystyrene involves breaking the vinyl double bond to form strong carbon-carbon single bonds, giving polystyrene its rigid structure.
Production Process Comparison
Styrene is a liquid monomer produced primarily through the catalytic dehydrogenation of ethylbenzene, serving as the essential building block for polystyrene. Polystyrene is synthesized via polymerization of styrene monomers using either suspension, bulk, or emulsion polymerization techniques, resulting in a solid plastic with versatile applications. Understanding the production process differences highlights how raw styrene transforms into durable polystyrene materials for your manufacturing needs.
Physical and Mechanical Properties
Styrene is a clear, colorless liquid monomer with a density of about 0.91 g/cm3 and a boiling point of 145degC, known for its volatility and ease of polymerization. Polystyrene, formed by the polymerization of styrene, exhibits rigid, glass-like characteristics with a density around 1.05 g/cm3, high tensile strength, and excellent insulating properties, making it widely used in packaging and insulation. Your choice between styrene and polystyrene depends on the desired mechanical strength and physical state, with polystyrene providing durable solid plastic forms ideal for structural applications.
Common Uses and Applications
Styrene is primarily used as a monomer in the production of polystyrene, which serves as a versatile plastic in packaging, insulation, and disposable consumer products. Polystyrene's lightweight, rigid structure makes it ideal for use in food containers, plastic cutlery, and protective packaging materials. Expanded polystyrene variants are widely employed in construction for thermal insulation and cushioning purposes.
Environmental Impact and Recycling
Styrene, a volatile organic compound used in manufacturing, poses significant environmental risks due to its toxicity and potential to contribute to air and water pollution. Polystyrene, derived from styrene, is widely criticized for its persistence in the environment, non-biodegradability, and challenges in recycling, leading to extensive accumulation in landfills and oceans. Advanced recycling methods like chemical recycling and pyrolysis show promise in converting polystyrene waste into reusable materials, reducing environmental impact but remain limited in global adoption.
Health and Safety Considerations
Styrene exposure poses significant health risks, including respiratory irritation, skin sensitization, and potential carcinogenicity as classified by the International Agency for Research on Cancer (IARC). In contrast, polystyrene, as a polymerized form of styrene, is generally considered inert and safe for use in food packaging, though it can release styrene monomers if improperly heated or damaged. Proper ventilation, protective equipment, and adherence to occupational exposure limits are critical when handling styrene to minimize health hazards associated with its volatile organic compounds.
Cost and Market Availability
Styrene, a key monomer, is generally more cost-effective and widely available in raw chemical markets compared to polystyrene, which requires additional polymerization processing. Polystyrene, as a finished polymer product, commands a higher price due to manufacturing complexity and application-specific grades. Market availability of styrene is robust with global production exceeding several million tons annually, whereas polystyrene availability depends on demand within packaging, insulation, and consumer goods industries.
Advantages and Disadvantages
Styrene offers advantages such as easy polymerization and versatility as a monomer for producing various plastics, but its toxicity and environmental hazards pose significant drawbacks. Polystyrene provides excellent insulation, lightweight properties, and cost-effectiveness, making it ideal for packaging and construction; however, it suffers from poor biodegradability and limited impact resistance. Understanding these pros and cons helps you choose the appropriate material for specific industrial or consumer applications.
Conclusion: Choosing Between Styrene and Polystyrene
Choosing between styrene and polystyrene depends on the intended application and material properties required. Styrene, a liquid monomer, serves as the precursor for polymerization, whereas polystyrene is the solid polymer used extensively in packaging, insulation, and consumer goods. Consider factors like mechanical strength, chemical resistance, and thermal stability to determine the optimal choice for manufacturing or design projects.
Styrene vs polystyrene Infographic
 libmatt.com
libmatt.com