Organic rubber, derived from natural sources like latex, offers superior elasticity and biodegradability, making it ideal for eco-friendly applications. Inorganic rubber, synthesized from petroleum-based materials, provides enhanced resistance to heat, chemicals, and aging, ensuring durability in industrial uses.
Table of Comparison
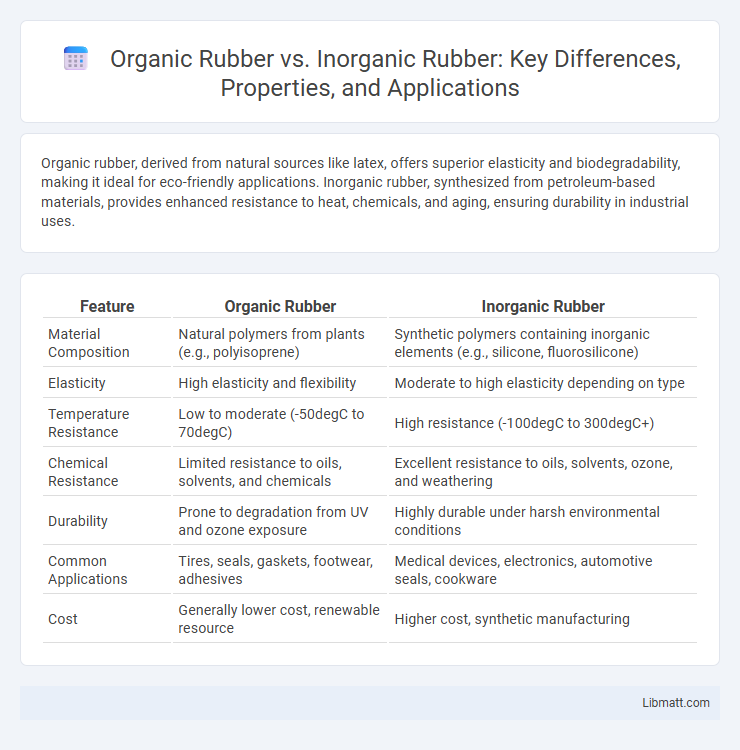
| Feature | Organic Rubber | Inorganic Rubber |
|---|---|---|
| Material Composition | Natural polymers from plants (e.g., polyisoprene) | Synthetic polymers containing inorganic elements (e.g., silicone, fluorosilicone) |
| Elasticity | High elasticity and flexibility | Moderate to high elasticity depending on type |
| Temperature Resistance | Low to moderate (-50degC to 70degC) | High resistance (-100degC to 300degC+) |
| Chemical Resistance | Limited resistance to oils, solvents, and chemicals | Excellent resistance to oils, solvents, ozone, and weathering |
| Durability | Prone to degradation from UV and ozone exposure | Highly durable under harsh environmental conditions |
| Common Applications | Tires, seals, gaskets, footwear, adhesives | Medical devices, electronics, automotive seals, cookware |
| Cost | Generally lower cost, renewable resource | Higher cost, synthetic manufacturing |
Introduction to Rubber: Organic vs Inorganic
Organic rubber, derived primarily from natural latex found in rubber trees, consists of long-chain hydrocarbons with natural polymer structures offering superior elasticity and resilience. Inorganic rubber, such as silicone and fluororubber, involves synthetic polymers composed of inorganic elements like silicon and fluorine, providing enhanced heat resistance, chemical stability, and durability under extreme conditions. Both types serve distinct applications based on their molecular composition and performance characteristics in industries like automotive, electronics, and medical devices.
Definition and Composition of Organic Rubber
Organic rubber refers to natural or synthetic elastomers derived primarily from carbon-based polymers composed of long chains of hydrocarbons, such as polyisoprene naturally found in latex from rubber trees. These materials exhibit high elasticity and resilience due to their molecular structure, which includes repeated organic monomers like isoprene units. Compared to inorganic rubber, organic rubber's composition relies on carbon-hydrogen bonds, contributing to its flexibility, biodegradability, and widespread use in tires, seals, and flexible products.
Key Characteristics of Inorganic Rubber
Inorganic rubber primarily consists of silicone and fluorosilicone materials, known for exceptional resistance to heat, chemicals, and ozone compared to organic rubber. It maintains flexibility and stability across a wide temperature range, from -60degC to 250degC, making it ideal for high-performance industrial applications. Its key characteristics include superior weather resistance, low gas permeability, and excellent dielectric properties, differentiating it significantly from organic rubbers like natural or synthetic polyisoprene.
Sources and Synthesis Processes
Organic rubber primarily derives from natural sources such as Hevea brasiliensis (rubber tree), where polyisoprene is biosynthesized through enzymatic polymerization of isoprene monomers. Inorganic rubber, often exemplified by silicone rubber, is synthesized through chemical processes involving the polymerization of siloxane monomers derived from silicon, oxygen, carbon, and hydrogen atoms. The natural origin of organic rubber contrasts with the synthetic pathways of inorganic rubber, highlighting distinct raw material bases and polymerization mechanisms essential for tailored material properties.
Mechanical Properties Comparison
Organic rubber, such as natural rubber and synthetic variants like styrene-butadiene rubber (SBR), typically exhibits superior elasticity, tensile strength, and abrasion resistance compared to inorganic rubber compounds. Inorganic rubbers, such as silicone and fluorosilicone, excel in heat resistance, chemical stability, and low-temperature flexibility but generally have lower tensile strength and elongation at break. The choice between organic and inorganic rubber depends on application-specific mechanical demands, balancing factors like durability, flexibility, and environmental resistance.
Chemical Resistance and Stability
Organic rubber, such as natural rubber or styrene-butadiene rubber (SBR), generally exhibits lower chemical resistance and stability when exposed to oils, solvents, and extreme temperatures. In contrast, inorganic rubber, like silicone or fluorosilicone rubber, offers superior chemical resistance, maintaining stability and elasticity in harsh environments including exposure to acids, alkalis, and high temperatures. The enhanced molecular structure of inorganic rubber provides greater durability and longevity in chemically aggressive applications.
Environmental Impact and Sustainability
Organic rubber, derived from natural latex harvested from Hevea brasiliensis trees, offers higher biodegradability and a lower carbon footprint compared to inorganic rubber made from synthetic polymers like styrene-butadiene or polyisoprene. The cultivation of organic rubber supports carbon sequestration and promotes soil health, reducing environmental degradation outcomes associated with petroleum-based synthetic rubber production. In contrast, inorganic rubber relies heavily on fossil fuels, generating greenhouse gas emissions and persistence in ecosystems, posing significant challenges to sustainability and long-term ecological balance.
Common Applications in Industry
Organic rubber, including natural rubber and synthetic polyisoprene, is commonly used in applications requiring high elasticity and resilience, such as tires, conveyor belts, and footwear soles. Inorganic rubber, like silicone and fluorosilicone, excels in environments demanding superior heat resistance, chemical stability, and electrical insulation, making it ideal for aerospace seals, medical devices, and automotive gaskets. Your selection depends on specific industry needs such as temperature tolerance, flexibility, and exposure to chemicals.
Cost Analysis and Market Trends
Organic rubber, primarily derived from natural sources such as latex from rubber trees, generally incurs higher production costs due to factors like cultivation, harvesting, and environmental susceptibility. Inorganic rubber, usually synthetic and produced from petrochemical compounds like styrene-butadiene or nitrile, offers cost advantages through large-scale industrial manufacturing and consistent quality, driving competitive pricing in various applications. Your decision between organic and inorganic rubber depends on balancing cost-efficiency with market demand trends, where growing sustainability concerns are gradually increasing the market share and value proposition of organic rubber despite its higher costs.
Future Prospects and Innovations in Rubber Technology
Organic rubber, primarily sourced from natural latex, is experiencing advancements through genetic engineering and sustainable harvesting methods to enhance elasticity and environmental compatibility. Inorganic rubber, such as silicone and synthetic variants, benefits from nanotechnology and polymer blending innovations that improve thermal stability and chemical resistance, expanding its applications in extreme environments. Future prospects in rubber technology emphasize hybrid materials combining organic and inorganic properties to achieve superior performance, durability, and eco-friendliness.
Organic rubber vs Inorganic rubber Infographic
 libmatt.com
libmatt.com