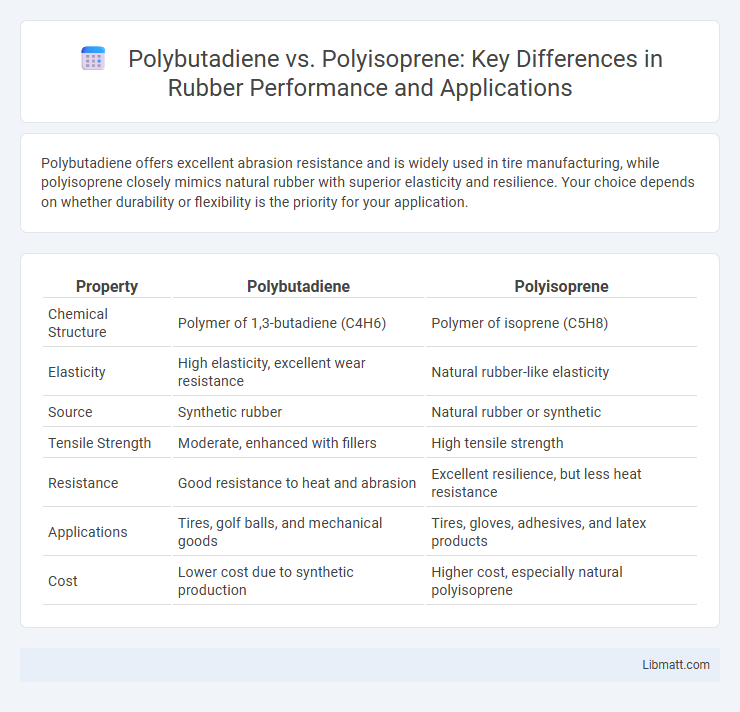
Polybutadiene offers excellent abrasion resistance and is widely used in tire manufacturing, while polyisoprene closely mimics natural rubber with superior elasticity and resilience. Your choice depends on whether durability or flexibility is the priority for your application.
Table of Comparison
| Property | Polybutadiene | Polyisoprene |
|---|---|---|
| Chemical Structure | Polymer of 1,3-butadiene (C4H6) | Polymer of isoprene (C5H8) |
| Elasticity | High elasticity, excellent wear resistance | Natural rubber-like elasticity |
| Source | Synthetic rubber | Natural rubber or synthetic |
| Tensile Strength | Moderate, enhanced with fillers | High tensile strength |
| Resistance | Good resistance to heat and abrasion | Excellent resilience, but less heat resistance |
| Applications | Tires, golf balls, and mechanical goods | Tires, gloves, adhesives, and latex products |
| Cost | Lower cost due to synthetic production | Higher cost, especially natural polyisoprene |
Introduction to Polybutadiene and Polyisoprene
Polybutadiene is a synthetic rubber known for its high resistance to abrasion and low-temperature flexibility, commonly used in tire manufacturing and industrial products. Polyisoprene, a natural or synthetic elastomer, closely mimics natural rubber with excellent elasticity and resilience, making it ideal for medical gloves and adhesives. The molecular structure of polybutadiene consists of polymerized 1,3-butadiene units, while polyisoprene polymerizes isoprene monomers, resulting in distinct physical and chemical properties.
Chemical Structure Comparison
Polybutadiene consists of repeating units derived from 1,3-butadiene, characterized by a backbone with alternating single and double carbon-carbon bonds, typically featuring cis-1,4, trans-1,4, and 1,2-vinyl configurations that influence its elasticity and resilience. Polyisoprene is composed of isoprene monomers with a similar polymer backbone but distinct methyl side groups attached to the carbon chain, primarily existing in a cis-1,4 configuration that closely mimics natural rubber's molecular arrangement. The presence of methyl groups in polyisoprene contributes to its higher crystallinity and tensile strength compared to polybutadiene, impacting their respective mechanical properties and applications.
Synthesis and Production Methods
Polybutadiene is synthesized primarily through anionic polymerization of 1,3-butadiene using initiators such as organolithium compounds, resulting in polymers with high cis-1,4 or trans-1,4 content depending on catalysts and conditions. Polyisoprene production employs coordination polymerization or anionic methods to polymerize isoprene monomers, where factors like temperature and catalyst type influence cis-1,4-polyisoprene yield, closely mimicking natural rubber structure. Industrial scale production of polybutadiene often utilizes emulsion polymerization for cost efficiency, while polyisoprene's synthetic routes aim for precise stereoregularity to replicate natural rubber properties.
Mechanical Properties
Polybutadiene exhibits excellent impact resistance and low hysteresis, making it ideal for applications requiring high durability and abrasion resistance, such as tires and golf balls. Polyisoprene offers superior elasticity and tensile strength, closely mimicking natural rubber properties, which enhances flexibility and resilience in products like gloves and balloons. The difference in microstructure between cis-1,4-polyisoprene and high-cis-polybutadiene significantly influences their mechanical performance, with polyisoprene providing better stretchability and polybutadiene delivering enhanced toughness.
Thermal Stability and Performance
Polybutadiene exhibits high thermal stability with resistance to heat degradation up to approximately 180degC, making it suitable for applications requiring durability under elevated temperatures. Polyisoprene, while offering excellent elasticity and abrasion resistance, generally shows lower thermal stability, degrading around 160degC. For performance in harsh thermal environments, polybutadiene outperforms polyisoprene due to its superior heat resistance and dimensional stability.
Applications in Industry
Polybutadiene is predominantly used in the automotive industry for manufacturing tires due to its high wear resistance and low rolling resistance, enhancing fuel efficiency. Polyisoprene closely mimics natural rubber, making it ideal for medical gloves, balloons, and adhesives where elasticity and biocompatibility are crucial. Both polymers are also utilized in the production of elastomers, but their distinct molecular structures dictate specific industrial applications based on performance requirements.
Environmental Impact and Sustainability
Polybutadiene and polyisoprene differ significantly in their environmental impact and sustainability profiles. Polyisoprene, often derived from natural rubber, is renewable and biodegradable, making it a more eco-friendly option compared to synthetic polybutadiene, which is petrochemical-based and less environmentally sustainable. You can reduce your ecological footprint by choosing polyisoprene products, which support renewable resources and generate less pollution during production.
Cost and Market Trends
Polybutadiene offers a lower cost advantage compared to polyisoprene, making it the preferred choice in high-volume applications such as tire manufacturing and industrial goods. Market trends indicate growing demand for polybutadiene due to its enhanced wear resistance and cost efficiency, while polyisoprene maintains a niche in high-performance and medical-grade products because of its superior elasticity and biocompatibility. The polybutadiene segment anticipates steady growth driven by automotive and manufacturing sectors, whereas polyisoprene's market expands in specialty applications with rising focus on sustainability and synthetic alternatives.
Advantages and Limitations
Polybutadiene offers superior abrasion resistance and low rolling resistance, making it ideal for tire manufacturing, while its limitation lies in lower elasticity compared to polyisoprene. Polyisoprene closely mimics natural rubber with excellent elasticity and resilience, but it tends to have poorer resistance to wear and weathering. Your choice depends on whether durability or elasticity is the priority for the specific application.
Future Outlook and Innovations
Polybutadiene and polyisoprene are undergoing significant advancements driven by sustainability and performance enhancements in the rubber industry. Innovations in bio-based synthesis and nanocomposite technology aim to improve durability and environmental impact, positioning these polymers as crucial materials for automotive tires and medical devices. Future developments prioritize optimizing polymer microstructure for tailored mechanical properties and increased recyclability, addressing evolving regulatory and market demands.
Polybutadiene vs Polyisoprene Infographic
 libmatt.com
libmatt.com