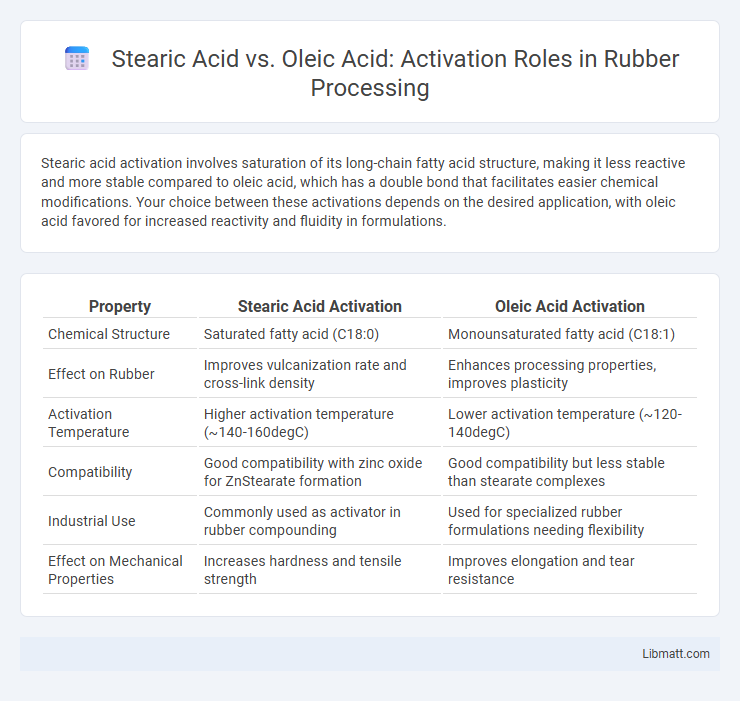
Stearic acid activation involves saturation of its long-chain fatty acid structure, making it less reactive and more stable compared to oleic acid, which has a double bond that facilitates easier chemical modifications. Your choice between these activations depends on the desired application, with oleic acid favored for increased reactivity and fluidity in formulations.
Table of Comparison
| Property | Stearic Acid Activation | Oleic Acid Activation |
|---|---|---|
| Chemical Structure | Saturated fatty acid (C18:0) | Monounsaturated fatty acid (C18:1) |
| Effect on Rubber | Improves vulcanization rate and cross-link density | Enhances processing properties, improves plasticity |
| Activation Temperature | Higher activation temperature (~140-160degC) | Lower activation temperature (~120-140degC) |
| Compatibility | Good compatibility with zinc oxide for ZnStearate formation | Good compatibility but less stable than stearate complexes |
| Industrial Use | Commonly used as activator in rubber compounding | Used for specialized rubber formulations needing flexibility |
| Effect on Mechanical Properties | Increases hardness and tensile strength | Improves elongation and tear resistance |
Introduction to Stearic Acid and Oleic Acid
Stearic acid, a saturated fatty acid with an 18-carbon chain, is commonly found in animal fats and certain plant oils, playing a vital role in cosmetics and manufacturing. Oleic acid, in contrast, is a monounsaturated fatty acid (C18:1) abundant in olive oil and other vegetable oils, known for its skin-conditioning and anti-inflammatory properties. The activation of these acids influences their chemical reactivity and functional applications, particularly in emulsification and synthesis processes.
Chemical Structure Comparison
Stearic acid and oleic acid differ primarily in their chemical structures: stearic acid is a saturated fatty acid with no double bonds, featuring a straight 18-carbon chain, while oleic acid is a monounsaturated fatty acid containing one cis double bond at the ninth carbon, introducing a kink in its 18-carbon chain. This structural difference influences their physical properties and reactivity during activation processes, with your reactions potentially favoring one based on the presence or absence of unsaturation. Understanding these molecular distinctions is crucial for optimizing chemical activation methods and tailoring applications in industries like cosmetics or pharmaceuticals.
Sources and Natural Occurrence
Stearic acid, a saturated fatty acid, primarily occurs in animal fats such as beef tallow and cocoa butter, as well as in some plant oils like shea butter. Oleic acid, a monounsaturated fatty acid, is abundantly found in olive oil, avocado oil, and various nuts, making it a key component of the Mediterranean diet. Both acids are critical components in natural lipid structures, influencing their activation and functional properties in biological and industrial applications.
Physical Properties Distinctions
Stearic acid exhibits a higher melting point (around 69-70degC) due to its saturated, long-chain structure, making it solid at room temperature, whereas oleic acid is a monounsaturated fatty acid with a melting point near 13-14degC, typically liquid at room temperature. The saturation level in stearic acid contributes to its greater chemical stability and lower reactivity in activation processes compared to the more reactive double bond present in oleic acid. Understanding these physical property distinctions is crucial for optimizing your activation method and enhancing the efficiency of subsequent chemical or industrial applications.
Metabolic Pathways and Activation Mechanisms
Stearic acid undergoes activation primarily through acyl-CoA synthetase enzymes, converting it to stearoyl-CoA, essential for its b-oxidation in mitochondria and elongation processes in the endoplasmic reticulum. Oleic acid is similarly activated to oleoyl-CoA by long-chain acyl-CoA synthetase, facilitating its incorporation into complex lipids and desaturation pathways catalyzed by stearoyl-CoA desaturase-1 (SCD1). Both fatty acids enter distinct metabolic pathways where activation governs their roles in energy production, membrane lipid composition, and signaling lipid biosynthesis.
Enzymatic Activation: Stearoyl-CoA vs Oleoyl-CoA
Stearic acid undergoes enzymatic activation primarily through conversion to stearoyl-CoA, facilitated by acyl-CoA synthetase enzymes that catalyze the ATP-dependent ligation of stearic acid to Coenzyme A. Oleic acid is similarly activated to oleoyl-CoA via the same enzyme family, with substrate specificity influencing the rate and affinity differences between saturated stearic acid and monounsaturated oleic acid. These activated acyl-CoA derivatives serve as essential intermediates in lipid metabolism, including b-oxidation, phospholipid biosynthesis, and membrane remodeling pathways.
Role in Cellular Functions
Stearic acid and oleic acid influence cellular functions through distinct lipid signaling pathways affecting membrane fluidity and protein interactions. Stearic acid promotes apoptosis and differentiation in various cell types, while oleic acid enhances cell proliferation and anti-inflammatory responses by modulating gene expression and receptor activity. These fatty acids' differential activation of enzymes such as protein kinase C and nuclear receptors crucially regulates cell growth, metabolism, and immune responses.
Industrial and Commercial Applications
Stearic acid and oleic acid differ significantly in their industrial and commercial applications due to their chemical properties; stearic acid, a saturated fatty acid, is widely used in the production of soaps, cosmetics, and candles because of its solid form and ability to provide hardness and stability. Oleic acid, an unsaturated fatty acid, is primarily utilized in the manufacture of lubricants, surfactants, and emulsifiers, benefiting from its liquid state and excellent solubility properties. Both acids are crucial in the production of activated derivatives for use in pharmaceuticals, food additives, and polymer manufacturing, where their unique activation processes impact product performance and functionality.
Health Impacts and Nutritional Implications
Stearic acid and oleic acid differ significantly in their health impacts and nutritional implications; stearic acid, a saturated fat found predominantly in animal fats and cocoa butter, has a neutral effect on LDL cholesterol levels, making it less harmful compared to other saturated fats. Oleic acid, a monounsaturated fat abundant in olive oil and avocados, promotes cardiovascular health by improving HDL cholesterol and reducing inflammation. Your diet benefits more from oleic acid's lipid profile enhancement, although moderate stearic acid intake does not elevate cardiovascular risk like other saturated fats.
Summary: Key Differences in Activation
Stearic acid and oleic acid differ significantly in activation due to their molecular structures; stearic acid is a saturated fatty acid with no double bonds, requiring higher energy for activation, while oleic acid is monounsaturated with one cis double bond that lowers activation energy. The presence of this double bond in oleic acid enhances reactivity and influences enzymatic activation pathways in metabolic processes. Your choice between these acids impacts reaction efficiency and biological function based on their distinct activation characteristics.
Stearic acid vs Oleic acid activation Infographic
 libmatt.com
libmatt.com