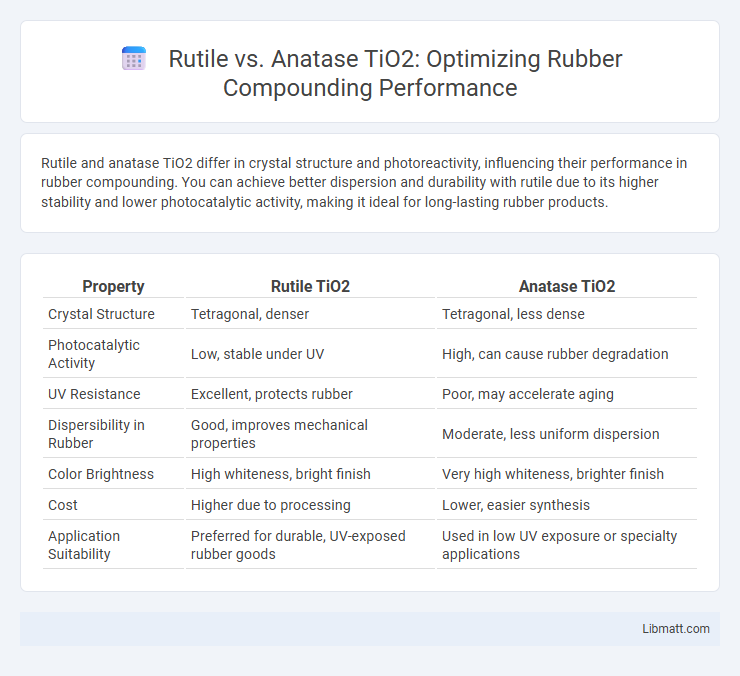
Rutile and anatase TiO2 differ in crystal structure and photoreactivity, influencing their performance in rubber compounding. You can achieve better dispersion and durability with rutile due to its higher stability and lower photocatalytic activity, making it ideal for long-lasting rubber products.
Table of Comparison
| Property | Rutile TiO2 | Anatase TiO2 |
|---|---|---|
| Crystal Structure | Tetragonal, denser | Tetragonal, less dense |
| Photocatalytic Activity | Low, stable under UV | High, can cause rubber degradation |
| UV Resistance | Excellent, protects rubber | Poor, may accelerate aging |
| Dispersibility in Rubber | Good, improves mechanical properties | Moderate, less uniform dispersion |
| Color Brightness | High whiteness, bright finish | Very high whiteness, brighter finish |
| Cost | Higher due to processing | Lower, easier synthesis |
| Application Suitability | Preferred for durable, UV-exposed rubber goods | Used in low UV exposure or specialty applications |
Introduction to TiO₂ in Rubber Compounding
TiO2 plays a crucial role in rubber compounding, enhancing properties such as opacity, brightness, and durability. Rutile TiO2 offers superior lightfastness and weather resistance, making it ideal for outdoor rubber applications, while anatase TiO2 provides higher photocatalytic activity beneficial for certain specialty rubber formulations. Your choice between rutile and anatase depends on the specific performance requirements and environmental exposure of the rubber product.
Overview of Rutile and Anatase Crystal Structures
Rutile and anatase are two primary polymorphs of titanium dioxide (TiO2) distinguished by their crystal structures, which significantly influence their performance in rubber compounding. Rutile exhibits a tetragonal crystal structure with a higher density and refractive index, providing excellent durability and UV resistance, making it ideal for reinforcing rubber materials. Anatase, also tetragonal but with a less dense and more open lattice, offers greater photocatalytic activity and dispersion properties, often enhancing the rubber's surface characteristics and aging resistance.
Key Physical and Chemical Differences
Rutile and anatase TiO2 differ primarily in crystal structure, with rutile exhibiting a tetragonal structure that offers higher density and greater refractive index, enhancing durability in rubber compounding. Chemically, rutile is more stable and resistant to photocatalytic degradation compared to anatase, making it preferable in high-performance rubber applications requiring enhanced UV resistance. Anatase presents higher surface area and greater catalytic activity, which can accelerate cross-linking reactions but may reduce long-term mechanical stability in rubber matrices.
Influence on Rubber Mechanical Properties
Rutile and anatase TiO2 differ significantly in their impact on rubber mechanical properties, with rutile providing superior reinforcement due to its higher refractive index and better dispersion characteristics. Rutile enhances tensile strength, abrasion resistance, and overall durability in rubber compounds, while anatase tends to offer lower mechanical performance but can improve processing properties. Your choice between these TiO2 forms will influence the balance of strength and flexibility in the final rubber product.
Impact on Aging and UV Resistance
Rutile TiO2 offers superior UV resistance in rubber compounding due to its higher refractive index and greater light absorption, effectively protecting polymers from photodegradation. Anatase TiO2, while more photocatalytically active, can accelerate polymer aging by generating reactive oxygen species under UV exposure. Selecting rutile over anatase ensures enhanced durability and longevity of rubber products in UV-intensive environments.
Color and Optical Properties in Rubber
Rutile and anatase TiO2 differ significantly in color and optical properties impacting rubber compounding, where rutile provides superior opacity and a brighter, whiter appearance. Rutile's higher refractive index enhances light scattering, improving rubber's visual brightness and durability under UV exposure. Your rubber formulations benefit from rutile's stability and color retention, making it ideal for applications demanding long-lasting whiteness and color vibrancy.
Dispersion and Processing Behavior
Rutile and anatase TiO2 exhibit distinct dispersion and processing behaviors in rubber compounding, where rutile offers superior light stability and better dispersion due to its surface treatment with silica or alumina, enhancing filler-polymer interaction. Anatase, with its higher photocatalytic activity, may lead to faster degradation but provides improved initial whiteness and brightness in the compound. Your choice between rutile and anatase TiO2 impacts processing efficiency and final product properties by balancing dispersion quality against UV resistance.
Cost and Availability Comparison
Rutile TiO2 is generally more expensive than anatase due to its superior photocatalytic stability and enhanced UV resistance, which are critical for rubber compounding applications. Anatase TiO2 offers a cost-effective alternative with sufficient performance for less demanding environments, benefiting manufacturers aiming to reduce material expenses. Your choice depends on balancing cost constraints with the specific performance requirements of the rubber formulation.
Environmental and Safety Considerations
Rutile TiO2 exhibits enhanced UV stability and lower photocatalytic activity compared to anatase, reducing oxidative degradation risks in rubber applications and improving polymer longevity. Anatase TiO2, known for higher photocatalytic reactivity, can generate reactive oxygen species that compromise rubber integrity and pose environmental concerns due to potential oxidative byproducts. Selecting rutile over anatase in rubber compounding minimizes environmental impact through reduced degradation and aligns with safer handling protocols by limiting oxidative hazards during production and end-use.
Selecting the Right TiO₂ Type for Rubber Applications
Rutile TiO2 is preferred in rubber compounding for its superior durability, weather resistance, and enhanced opacity, making it ideal for outdoor and heavy-duty rubber products. Anatase TiO2 offers higher photocatalytic activity but lower stability, which can lead to faster degradation in rubber applications exposed to UV light. Selecting the right TiO2 type depends on balancing desired properties such as UV resistance, color strength, and processing conditions specific to the rubber application.
Rutile vs Anatase TiO₂ in rubber compounding Infographic
 libmatt.com
libmatt.com