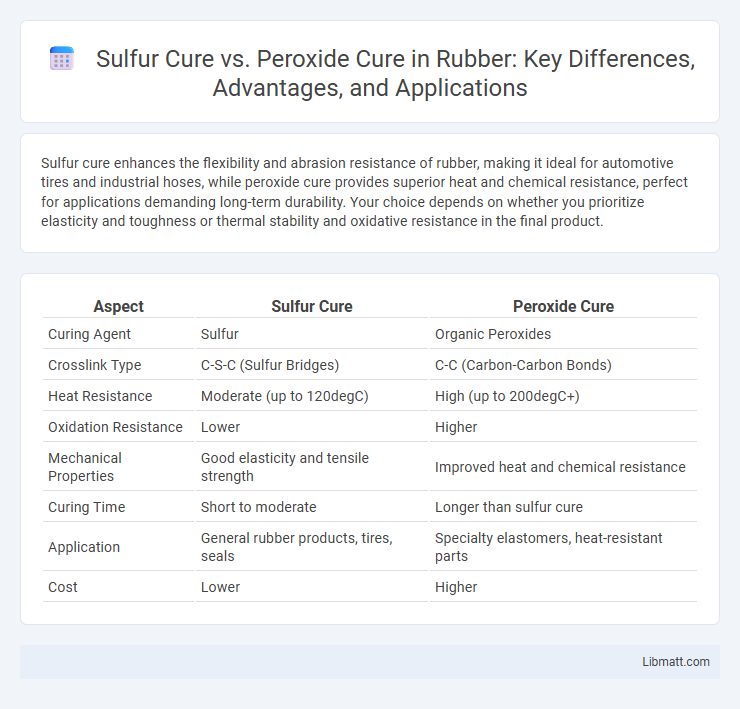
Sulfur cure enhances the flexibility and abrasion resistance of rubber, making it ideal for automotive tires and industrial hoses, while peroxide cure provides superior heat and chemical resistance, perfect for applications demanding long-term durability. Your choice depends on whether you prioritize elasticity and toughness or thermal stability and oxidative resistance in the final product.
Table of Comparison
| Aspect | Sulfur Cure | Peroxide Cure |
|---|---|---|
| Curing Agent | Sulfur | Organic Peroxides |
| Crosslink Type | C-S-C (Sulfur Bridges) | C-C (Carbon-Carbon Bonds) |
| Heat Resistance | Moderate (up to 120degC) | High (up to 200degC+) |
| Oxidation Resistance | Lower | Higher |
| Mechanical Properties | Good elasticity and tensile strength | Improved heat and chemical resistance |
| Curing Time | Short to moderate | Longer than sulfur cure |
| Application | General rubber products, tires, seals | Specialty elastomers, heat-resistant parts |
| Cost | Lower | Higher |
Introduction to Rubber Vulcanization
Rubber vulcanization is a chemical process that improves the elasticity, strength, and durability of rubber through the formation of cross-links between polymer chains. Sulfur cure is the most common vulcanization method, creating polysulfide linkages that enhance flexibility and tensile strength, particularly suitable for natural and synthetic rubbers like NR and SBR. Peroxide cure involves organic peroxides generating carbon-carbon cross-links, offering superior heat and aging resistance, ideal for specialty rubbers such as EPDM and silicone.
Overview of Sulfur Cure Process
The sulfur cure process involves the cross-linking of rubber molecules using sulfur, which forms sulfur bridges between polymer chains, enhancing elasticity and durability. This method is widely used in the vulcanization of natural rubber and various synthetic rubbers, providing superior mechanical strength and resistance to abrasion. Optimal curing conditions, such as temperature and sulfur concentration, are crucial to achieving desired material properties and preventing overcuring or scorching.
Overview of Peroxide Cure Process
The peroxide cure process involves the use of organic peroxides as curing agents, which generate free radicals to initiate cross-linking in rubber compounds, resulting in enhanced heat resistance and aging stability. This method offers superior mechanical properties and improved resistance to ozone, oxygen, and fatigue compared to sulfur curing. Peroxide curing is particularly effective for elastomers like EPDM, silicones, and fluoroelastomers, providing cleaner vulcanization with fewer by-products.
Key Differences in Crosslinking Mechanisms
Sulfur cure systems create crosslinks through sulfur atoms forming polysulfidic, disulfidic, or monosulfidic bridges between polymer chains, resulting in flexible and heat-resistant rubber products. Peroxide cure mechanisms rely on free radical generation, causing carbon-carbon bonds to form directly, producing thermally stable and chemically resistant elastomers. Understanding these crosslinking differences helps you select the optimal curing method for specific performance requirements in rubber manufacturing.
Performance and Physical Properties Comparison
Sulfur cure systems offer superior tensile strength and elongation, making them ideal for rubber applications requiring elasticity and toughness, while peroxide cure provides enhanced heat resistance and better aging properties due to more stable carbon-carbon crosslinks. Sulfur-cured rubbers typically exhibit higher resilience and better compression set in dynamic applications, whereas peroxide-cured elastomers demonstrate improved chemical resistance and lower hysteresis, contributing to durability under thermal stress. Performance trade-offs include sulfur cure's faster cure time and cost efficiency versus peroxide cure's ability to retain physical properties over prolonged exposure to heat and oxidative environments.
Heat and Aging Resistance Analysis
Peroxide cure systems exhibit superior heat and aging resistance compared to sulfur cure methods due to their stable carbon-carbon crosslinks that withstand thermal degradation and oxidation. Sulfur cure networks, while providing superior dynamic properties, tend to deteriorate faster under prolonged heat exposure because of polysulfidic bonds that break down easily. In high-temperature applications, peroxide-cured elastomers maintain mechanical integrity and resist aging effects significantly better than sulfur-cured counterparts.
Application Suitability: Sulfur vs Peroxide
Sulfur cure is ideal for natural rubber and blends used in automotive tires, footwear, and general industrial goods due to its strong cross-linking and flexibility. Peroxide cure suits synthetic rubbers like EPDM, silicone, and fluoroelastomers, providing superior heat, chemical, and ozone resistance ideal for seals, hoses, and medical devices. Choosing the right curing system enhances Your product's performance by aligning the rubber type with the application environment.
Environmental and Safety Considerations
Sulfur cure systems release sulfur dioxide and other hazardous gases during vulcanization, posing environmental and health risks, while peroxide cure systems generate fewer toxic emissions and have a lower environmental impact. The peroxide cure process reduces the formation of carcinogenic nitrosamines, enhancing workplace safety for your manufacturing team. Choosing peroxide cure technology can minimize your facility's ecological footprint while ensuring safer handling and disposal procedures.
Cost Implications of Curing Systems
Sulfur cure systems generally offer lower upfront costs and are more cost-effective for large-scale rubber production due to simpler formulation and processing. Peroxide cure systems, while typically more expensive because of higher raw material costs and specialized equipment requirements, provide enhanced heat resistance and mechanical properties. Your choice between sulfur and peroxide curing will significantly impact overall production expenses and long-term product performance.
Choosing the Right Vulcanization Method
Choosing the right vulcanization method depends on the specific application requirements and rubber compound involved. Sulfur cure provides excellent mechanical properties and is ideal for natural rubber and styrene-butadiene rubber, whereas peroxide cure offers superior heat and chemical resistance suited for EPDM and silicone rubbers. Understanding the trade-offs between crosslink density, aging stability, and environmental exposure ensures optimal performance and longevity of the vulcanized product.
Sulfur Cure vs Peroxide Cure Infographic
 libmatt.com
libmatt.com