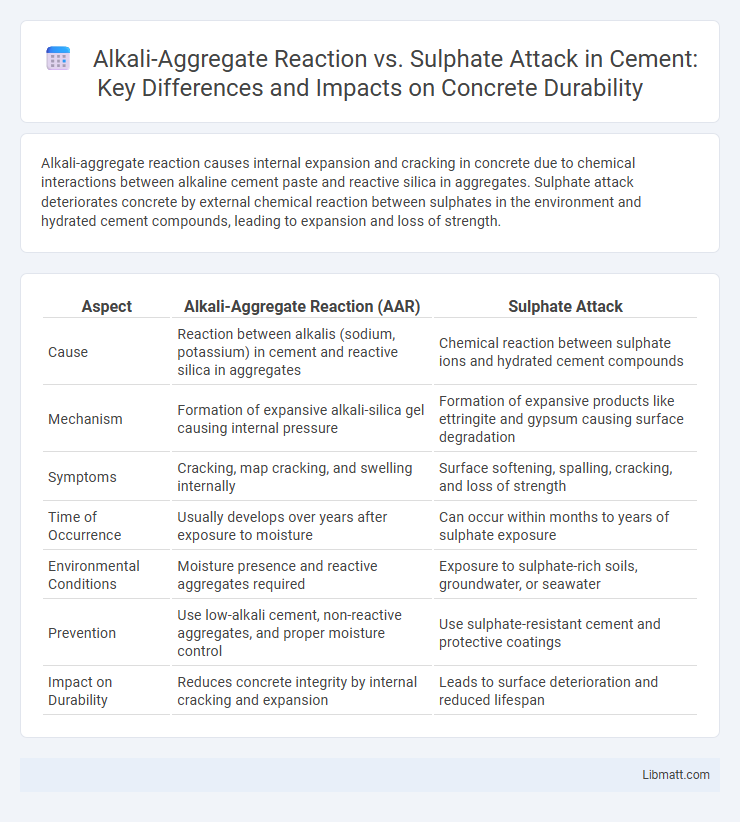
Alkali-aggregate reaction causes internal expansion and cracking in concrete due to chemical interactions between alkaline cement paste and reactive silica in aggregates. Sulphate attack deteriorates concrete by external chemical reaction between sulphates in the environment and hydrated cement compounds, leading to expansion and loss of strength.
Table of Comparison
| Aspect | Alkali-Aggregate Reaction (AAR) | Sulphate Attack |
|---|---|---|
| Cause | Reaction between alkalis (sodium, potassium) in cement and reactive silica in aggregates | Chemical reaction between sulphate ions and hydrated cement compounds |
| Mechanism | Formation of expansive alkali-silica gel causing internal pressure | Formation of expansive products like ettringite and gypsum causing surface degradation |
| Symptoms | Cracking, map cracking, and swelling internally | Surface softening, spalling, cracking, and loss of strength |
| Time of Occurrence | Usually develops over years after exposure to moisture | Can occur within months to years of sulphate exposure |
| Environmental Conditions | Moisture presence and reactive aggregates required | Exposure to sulphate-rich soils, groundwater, or seawater |
| Prevention | Use low-alkali cement, non-reactive aggregates, and proper moisture control | Use sulphate-resistant cement and protective coatings |
| Impact on Durability | Reduces concrete integrity by internal cracking and expansion | Leads to surface deterioration and reduced lifespan |
Understanding Alkali-Aggregate Reaction
Alkali-aggregate reaction (AAR) occurs when alkalis in cement react with reactive silica in aggregates, forming an expansive gel that causes cracking and structural damage in concrete. Sulphate attack, in contrast, involves sulphate ions penetrating concrete and reacting with hydration products, leading to expansion and deterioration primarily through ettringite formation. Understanding AAR is crucial for diagnosing concrete distress and implementing preventive measures, ensuring your structures maintain durability and integrity.
What Is Sulphate Attack in Concrete?
Sulphate attack in concrete occurs when sulphate ions from external sources penetrate the concrete and react with the hydrated compounds, primarily calcium aluminate hydrates, leading to the formation of expansive products like ettringite and gypsum. This chemical reaction causes swelling, cracking, and loss of strength, compromising the durability and structural integrity of concrete. Sulphate attack is commonly found in soils, groundwater, and industrial environments with high sulphate concentrations.
Causes of Alkali-Aggregate Reaction
Alkali-aggregate reaction occurs when reactive silica in aggregates interacts with alkali hydroxides in concrete pore water, forming an expansive gel that leads to cracking. High alkali content from cement and moisture availability worsen this internal chemical reaction. You can mitigate these effects by selecting non-reactive aggregates and using low-alkali cement to prevent structural damage.
Mechanisms Behind Sulphate Attack
Sulphate attack occurs when sulphate ions penetrate concrete and react with calcium hydroxide, forming expansive products like ettringite and gypsum, leading to concrete expansion and cracking. This chemical interaction disrupts the cement matrix and weakens the structural integrity over time. Understanding the roles of external sulphate sources and internal cement composition is crucial for predicting and mitigating sulphate-induced deterioration in concrete structures.
Symptoms and Effects on Concrete Structures
Alkali-aggregate reaction (AAR) manifests through cracking, swelling, and loss of strength in concrete due to the expansion of gel formed by the reaction between alkali hydroxides and reactive aggregates. Sulphate attack exhibits symptoms such as surface scaling, spalling, and softening of the concrete matrix caused by chemical reactions between sulphate ions and hydrated cement compounds, leading to expansion and cracking. Both deterioration mechanisms compromise structural integrity, reduce durability, and necessitate timely intervention to prevent failure.
Key Differences Between AAR and Sulphate Attack
Alkali-aggregate reaction (AAR) primarily occurs when reactive silica in aggregates reacts with alkali hydroxides in concrete, leading to expansive gel formation and cracking. Sulphate attack involves the intrusion of sulphate ions from external sources, causing chemical reactions with cement hydration products such as calcium aluminate hydrates, which form expansive products like ettringite, resulting in concrete deterioration. The main differences between AAR and sulphate attack lie in their causative agents--internal alkali-aggregate interactions versus external sulphate ion ingress--and the types of expansive compounds formed, with AAR producing alkali-silica gel and sulphate attack generating ettringite or gypsum.
Testing Methods for Detection
Alkali-aggregate reaction is commonly detected using petrographic analysis, accelerated mortar bar tests (ASTM C1260), and concrete prism tests (ASTM C1293) that measure expansion due to gel formation. Sulphate attack is identified through chemical analysis of sulfate ions, standardized sulfate exposure tests such as ASTM C1012, and monitoring of concrete expansion and pH changes over time. Both testing methods emphasize long-term durability evaluation to predict deterioration in concrete structures.
Prevention Strategies for Both Reactions
Prevention strategies for alkali-aggregate reaction include using low-alkali cement, incorporating supplementary cementitious materials like fly ash or slag, and selecting non-reactive aggregates to minimize expansion and cracking. Sulphate attack prevention involves using sulphate-resistant cement, ensuring proper concrete mix design with low permeability, and adequate curing to protect the reinforcement from chemical degradation. Your choice of quality materials and proper construction techniques plays a crucial role in mitigating both alkali-aggregate reaction and sulphate attack in concrete structures.
Repair and Mitigation Approaches
Effective repair and mitigation approaches for alkali-aggregate reaction (AAR) primarily involve removing or reducing alkali content through chemical treatments such as lithium nitrate application, and replacing affected concrete to prevent further expansion and cracking. Sulphate attack mitigation focuses on using sulphate-resistant cement types like Type V Portland cement and applying protective coatings to concrete surfaces to limit exposure to sulphate-rich environments. Your maintenance strategy should prioritize early detection and selection of appropriate repair materials tailored to the specific deterioration mechanism for long-term durability.
Long-Term Impacts on Structural Durability
Alkali-aggregate reaction (AAR) causes gradual expansion and cracking in concrete due to the formation of expansive gels, compromising structural durability over decades. Sulphate attack leads to chemical deterioration by forming gypsum and ettringite, resulting in severe spalling and loss of strength especially in sulfate-rich environments. Your structure's long-term resilience depends on identifying and mitigating these distinct degradation mechanisms to prevent costly repairs and failures.
Alkali-aggregate reaction vs Sulphate attack Infographic
 libmatt.com
libmatt.com