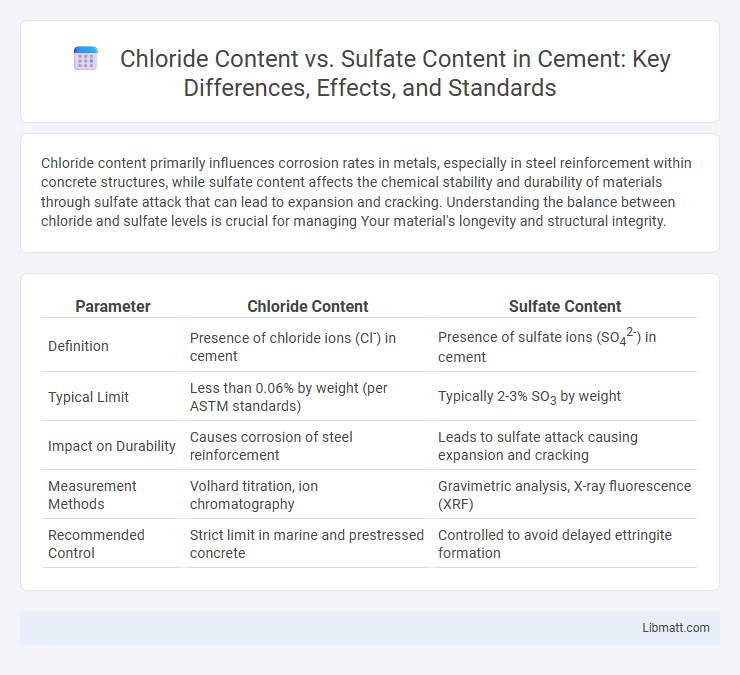
Chloride content primarily influences corrosion rates in metals, especially in steel reinforcement within concrete structures, while sulfate content affects the chemical stability and durability of materials through sulfate attack that can lead to expansion and cracking. Understanding the balance between chloride and sulfate levels is crucial for managing Your material's longevity and structural integrity.
Table of Comparison
| Parameter | Chloride Content | Sulfate Content |
|---|---|---|
| Definition | Presence of chloride ions (Cl-) in cement | Presence of sulfate ions (SO42-) in cement |
| Typical Limit | Less than 0.06% by weight (per ASTM standards) | Typically 2-3% SO3 by weight |
| Impact on Durability | Causes corrosion of steel reinforcement | Leads to sulfate attack causing expansion and cracking |
| Measurement Methods | Volhard titration, ion chromatography | Gravimetric analysis, X-ray fluorescence (XRF) |
| Recommended Control | Strict limit in marine and prestressed concrete | Controlled to avoid delayed ettringite formation |
Introduction to Chloride and Sulfate Compounds
Chloride and sulfate compounds are essential inorganic anions found in various natural and industrial processes, with chloride typically originating from sodium chloride (NaCl) and sulfate commonly derived from sulfuric acid (H2SO4) or gypsum (CaSO4*2H2O). Chloride content influences factors such as corrosion in metal structures and taste in water, while sulfate content impacts water hardness and can contribute to scaling in pipes and boiler systems. Monitoring and controlling chloride and sulfate concentrations are critical in water quality management and chemical manufacturing to prevent operational issues and ensure compliance with environmental standards.
Chemical Properties of Chlorides and Sulfates
Chlorides are highly soluble salts that dissociate easily in water, releasing chloride ions (Cl-) which contribute to electrical conductivity and corrosion in metals. Sulfates, containing the sulfate ion (SO42-), typically exhibit lower solubility compared to chlorides and can precipitate under certain conditions, affecting water hardness and scaling. Understanding the chemical properties of chlorides and sulfates helps you manage their impact on industrial processes and water quality.
Sources of Chloride and Sulfate Contamination
Chloride contamination primarily originates from sources such as seawater intrusion, agricultural runoff containing fertilizers, road salt application, and industrial discharges. Sulfate contamination is often linked to natural mineral dissolution, including gypsum and pyrite oxidation, as well as anthropogenic sources like mining activities and acid mine drainage. Understanding these sources is critical for water quality management and environmental remediation efforts.
Environmental Impact: Chloride vs Sulfate
Chloride content in water can lead to soil salinization, harming plant growth and disrupting aquatic ecosystems by increasing water toxicity for freshwater organisms. Sulfate content, while less toxic than chloride, can contribute to the acidification of water bodies, affecting aquatic life and altering nutrient availability. High levels of both chloride and sulfate demand careful monitoring to mitigate their detrimental environmental impacts on terrestrial and aquatic habitats.
Health Effects of Chloride and Sulfate Exposure
Exposure to high chloride content in water can lead to increased blood pressure and potential kidney damage, while excessive sulfate intake may cause gastrointestinal distress and dehydration. Chronic ingestion of elevated sulfate levels is particularly concerning for infants and individuals with compromised digestive systems. Understanding the health risks associated with chloride and sulfate exposure is crucial for water quality regulation and public health safety.
Analytical Methods for Chloride and Sulfate Measurement
Accurate determination of chloride and sulfate content is essential in water quality analysis, with ion chromatography and titrimetric methods being the most reliable analytical techniques. Ion chromatography offers precise quantification by separating ions based on their interaction with resin columns, while argentometric titration using silver nitrate specifically measures chloride concentration. Your choice of method depends on the required detection limits and sample matrix, ensuring precise monitoring of chloride and sulfate levels in environmental or industrial samples.
Regulatory Standards for Chloride and Sulfate Levels
Regulatory standards for chloride and sulfate levels in water vary globally, with organizations like the EPA setting maximum contaminant levels (MCLs) of 250 mg/L for chloride and 250 mg/L for sulfate in drinking water. These limits aim to prevent taste issues, corrosion, and potential health effects associated with high concentrations. Compliance with these standards ensures safe water quality for industrial, agricultural, and residential uses.
Effects on Infrastructure: Corrosion and Scaling
High chloride content in water accelerates corrosion of metal infrastructure by breaking down passive oxide layers, leading to increased risk of structural damage in pipelines and reinforcement bars. Elevated sulfate levels contribute to scaling by forming insoluble salts such as calcium sulfate, which can clog pipes and reduce flow efficiency. The combined presence of chlorides and sulfates exacerbates deterioration, necessitating accurate monitoring to prevent costly maintenance and ensure infrastructure longevity.
Comparative Analysis: Chloride Content vs Sulfate Content
Chloride content is typically higher in seawater, averaging around 19,000 mg/L, whereas sulfate content generally hovers near 2,700 mg/L, making chloride the dominant anion in marine environments. In groundwater and industrial wastewater, sulfate concentrations can vary widely from a few mg/L up to several thousands, often influenced by geological formations and pollution sources. Understanding the comparative levels of chloride and sulfate is crucial for water quality assessment, corrosion potential evaluation, and regulatory compliance in environmental management.
Mitigation Strategies and Removal Techniques
Mitigation strategies for chloride and sulfate content involve source control through the use of corrosion inhibitors and pH adjustment to reduce their impact on concrete and soil stability. Removal techniques include ion exchange, membrane filtration, and precipitation methods that effectively lower chloride and sulfate concentrations in water and wastewater treatment processes. Advanced adsorption materials such as activated carbon and specific resins also enhance the selective removal of these ions to protect infrastructure and prevent environmental contamination.
Chloride content vs Sulfate content Infographic
 libmatt.com
libmatt.com