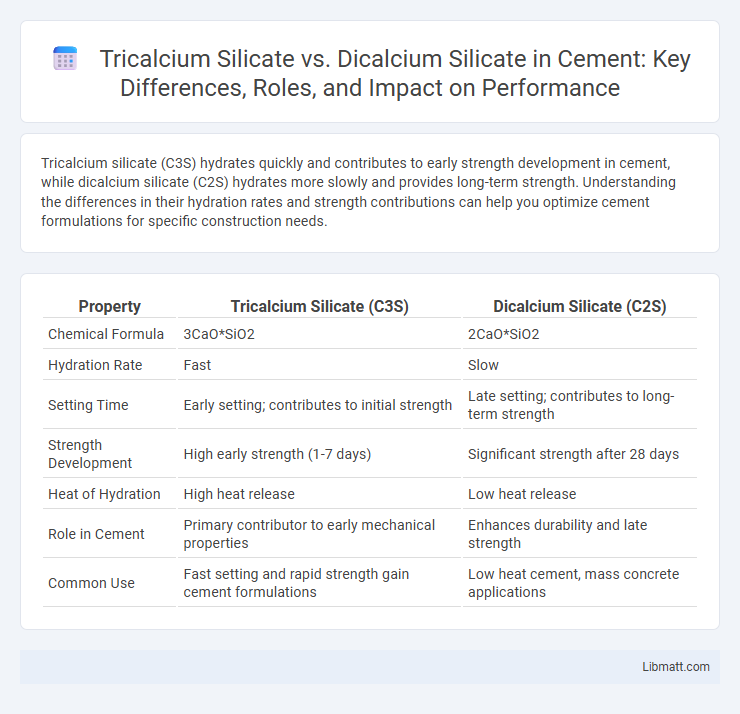
Tricalcium silicate (C3S) hydrates quickly and contributes to early strength development in cement, while dicalcium silicate (C2S) hydrates more slowly and provides long-term strength. Understanding the differences in their hydration rates and strength contributions can help you optimize cement formulations for specific construction needs.
Table of Comparison
| Property | Tricalcium Silicate (C3S) | Dicalcium Silicate (C2S) |
|---|---|---|
| Chemical Formula | 3CaO*SiO2 | 2CaO*SiO2 |
| Hydration Rate | Fast | Slow |
| Setting Time | Early setting; contributes to initial strength | Late setting; contributes to long-term strength |
| Strength Development | High early strength (1-7 days) | Significant strength after 28 days |
| Heat of Hydration | High heat release | Low heat release |
| Role in Cement | Primary contributor to early mechanical properties | Enhances durability and late strength |
| Common Use | Fast setting and rapid strength gain cement formulations | Low heat cement, mass concrete applications |
Introduction to Calcium Silicates
Tricalcium silicate (C3S) and dicalcium silicate (C2S) are key compounds found in Portland cement, crucial for its hydration and strength development. Tricalcium silicate hydrates rapidly, providing early strength within days, while dicalcium silicate hydrates slowly, contributing to long-term durability over months. These calcium silicates influence cement's mechanical properties, setting time, and resistance to chemical attack.
Chemical Structure: Tricalcium vs Dicalcium Silicate
Tricalcium silicate (Ca3SiO5) and dicalcium silicate (Ca2SiO4) differ primarily in their chemical structure, where tricalcium silicate contains three calcium ions bonded to one silicate ion, resulting in higher calcium content per formula unit. This structural difference influences their hydration rates, with tricalcium silicate hydrating faster and contributing to early strength development in cementitious materials. Dicalcium silicate's lower calcium ratio causes it to hydrate more slowly, providing long-term strength and durability in cement formulations.
Sources and Natural Occurrence
Tricalcium silicate (C3S) primarily originates from high-temperature processes involving limestone and clay, commonly found in Portland cement clinkers. Dicalcium silicate (C2S) occurs naturally in minerals like belite within certain types of meteorites and some limestone deposits. Your understanding of their distinct geological sources influences material selection in construction and cement manufacturing.
Synthesis and Manufacturing Processes
Tricalcium silicate (C3S) and dicalcium silicate (C2S) are synthesized through high-temperature solid-state reactions involving calcium carbonate and silica sources, typically processed in rotary kilns at temperatures between 1400-1500degC for C3S and around 1250-1350degC for C2S. The precise control of kiln temperature and residence time governs the phase composition, with C3S forming via faster reactions and higher temperatures, while C2S requires prolonged cooling to stabilize its crystalline structure. Advanced manufacturing techniques include coprecipitation and sol-gel methods to enhance homogeneity, but industrial production primarily relies on clinker burning and subsequent grinding for cement applications.
Physical Properties Comparison
Tricalcium silicate and dicalcium silicate differ significantly in physical properties, with tricalcium silicate exhibiting higher early strength and faster hydration rates compared to dicalcium silicate, which contributes to slower strength development over time. The density of tricalcium silicate is approximately 3.16 g/cm3, while dicalcium silicate has a slightly lower density around 2.9 g/cm3, affecting their respective volumes and mass in cement formulations. Understanding these differences helps optimize Your material selection for applications requiring specific setting times and strength performances.
Role in Cement Chemistry
Tricalcium silicate (C3S) plays a crucial role in cement chemistry by providing early strength development through rapid hydration and calcium silicate hydrate formation. Dicalcium silicate (C2S) hydrates more slowly, contributing to long-term strength and durability in cement. Understanding these compounds helps you optimize cement properties for specific construction applications.
Hydration Reaction Mechanisms
Tricalcium silicate (C3S) hydrates rapidly, producing calcium silicate hydrate (C-S-H) gel and calcium hydroxide, which contribute to early strength development in cement. Dicalcium silicate (C2S) hydrates more slowly, generating C-S-H gel over an extended period, thereby enhancing long-term strength and durability. Understanding the distinct hydration reaction mechanisms of C3S and C2S allows you to optimize concrete mix designs for specific performance requirements.
Impact on Mechanical Strength
Tricalcium silicate (C3S) contributes significantly to the early strength development in cement due to its rapid hydration and high calcium silicate content. Dicalcium silicate (C2S) hydrates more slowly, providing strength gain primarily at later stages, improving the long-term durability of concrete. The balance between C3S and C2S in cement composition directly influences both the initial mechanical strength and the sustained load-bearing capacity of construction materials.
Long-term Durability in Concrete
Tricalcium silicate (C3S) hydrates rapidly, providing early strength and contributing to initial concrete durability, while dicalcium silicate (C2S) hydrates slowly, enhancing long-term strength and stability by forming denser calcium silicate hydrate (C-S-H) gel over time. C2S's prolonged reaction ensures sustained durability in concrete structures exposed to harsh environmental conditions, reducing permeability and improving resistance to chemical attack. Understanding the balance of C3S and C2S in your concrete mix can optimize long-term performance and structural integrity.
Environmental and Economic Considerations
Tricalcium silicate (C3S) offers faster strength development, reducing construction time and associated labor costs, while its higher energy consumption during production increases environmental impact compared to dicalcium silicate (C2S). Dicalcium silicate contributes to longer-term strength with lower kiln temperatures required, resulting in reduced CO2 emissions and energy usage, making it more eco-friendly and cost-effective for sustainable construction projects. Understanding the balance between your project's timeline and environmental goals can guide material selection toward both economic savings and lower carbon footprint.
Tricalcium Silicate vs Dicalcium Silicate Infographic
 libmatt.com
libmatt.com