Flocculation and coagulation are essential water treatment processes that work together to remove suspended particles; coagulation destabilizes and neutralizes charges on particles, while flocculation gently mixes the water to form larger aggregates called flocs. Understanding the difference helps optimize your treatment system for clearer, cleaner water.
Table of Comparison
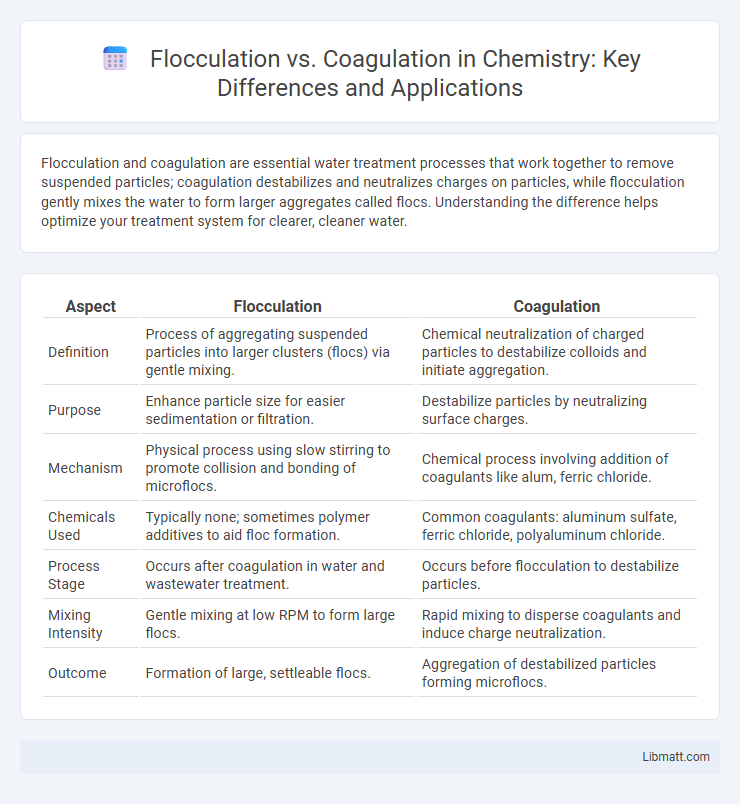
| Aspect | Flocculation | Coagulation |
|---|---|---|
| Definition | Process of aggregating suspended particles into larger clusters (flocs) via gentle mixing. | Chemical neutralization of charged particles to destabilize colloids and initiate aggregation. |
| Purpose | Enhance particle size for easier sedimentation or filtration. | Destabilize particles by neutralizing surface charges. |
| Mechanism | Physical process using slow stirring to promote collision and bonding of microflocs. | Chemical process involving addition of coagulants like alum, ferric chloride. |
| Chemicals Used | Typically none; sometimes polymer additives to aid floc formation. | Common coagulants: aluminum sulfate, ferric chloride, polyaluminum chloride. |
| Process Stage | Occurs after coagulation in water and wastewater treatment. | Occurs before flocculation to destabilize particles. |
| Mixing Intensity | Gentle mixing at low RPM to form large flocs. | Rapid mixing to disperse coagulants and induce charge neutralization. |
| Outcome | Formation of large, settleable flocs. | Aggregation of destabilized particles forming microflocs. |
Introduction to Flocculation and Coagulation
Flocculation and coagulation are essential processes in water treatment used to remove suspended particles and impurities. Coagulation involves the addition of chemicals, such as aluminum sulfate or ferric chloride, to destabilize and aggregate fine particles, while flocculation gently mixes the water to form larger clumps called flocs. These combined processes enhance sedimentation efficiency by creating particles large enough to settle quickly, improving overall water clarity and quality.
Definition of Coagulation
Coagulation is a chemical process in water treatment where coagulants such as aluminum sulfate or ferric chloride are added to destabilize suspended particles, causing them to aggregate into larger clumps. This process neutralizes the charges on particles, allowing them to come together and form microflocs that are easier to remove. Understanding coagulation is essential for optimizing your water purification system before subsequent flocculation and sedimentation steps.
Definition of Flocculation
Flocculation is the gentle process that causes suspended particles in a liquid to clump together, forming larger aggregates called flocs, which can then be easily separated. It follows coagulation, where chemicals neutralize the charges on particles to destabilize them, making flocculation essential for effective water and wastewater treatment. Your understanding of flocculation helps optimize sedimentation and filtration systems by improving particle removal efficiency.
Key Differences Between Coagulation and Flocculation
Coagulation involves the neutralization of particle charges to destabilize colloidal suspensions, typically using chemical coagulants such as aluminum sulfate or ferric chloride. Flocculation follows coagulation and consists of gentle mixing to promote the aggregation of these destabilized particles into larger flocs for easier removal. The main difference lies in their mechanisms: coagulation neutralizes charges, while flocculation physically promotes particle collision and bond formation.
Chemical Agents Used in Coagulation and Flocculation
Chemical agents used in coagulation typically include aluminum sulfate (alum), ferric chloride, and polyaluminum chloride, which neutralize the charges on suspended particles to facilitate their aggregation. Flocculation involves the use of polymers such as polyacrylamides and natural organic polymers like chitosan, which promote the formation of larger, settleable flocs by bridging particles together. Understanding the specific chemical agents applied in your water treatment processes can optimize the removal of turbidity and enhance overall efficiency.
Step-by-Step Process Comparison
Coagulation begins by adding chemical coagulants like aluminum sulfate, neutralizing particle charges and destabilizing suspended solids, while flocculation gently mixes water to encourage particle collisions and form larger aggregates called flocs. During coagulation, rapid mixing is critical for uniform distribution of coagulants, whereas flocculation involves slow, gentle stirring to allow flocs to grow without breaking apart. Understanding the step-by-step differences between these processes helps optimize water treatment systems for improved sediment removal and clearer water quality.
Factors Influencing Flocculation and Coagulation
Temperature, pH, and the presence of suspended particles significantly influence flocculation and coagulation efficiency. Optimal flocculant and coagulant dosages depend on water chemistry, including ionic strength and organic matter content. Your treatment process performance improves when these factors are carefully controlled to enhance particle aggregation and sedimentation.
Applications in Water and Wastewater Treatment
Flocculation and coagulation are critical processes in water and wastewater treatment used to remove suspended solids and contaminants. Coagulation destabilizes colloidal particles by adding chemicals such as alum or ferric chloride, enabling smaller particles to clump together. Flocculation follows by gently mixing these particles into larger flocs that can be easily separated through sedimentation or filtration, improving water clarity and quality.
Advantages and Limitations of Each Method
Flocculation offers the advantage of forming larger, more easily settled flocs through gentle mixing, making it effective for removing suspended solids in water treatment, but it requires precise control of mixing speed and chemical dosage to avoid breaking flocs. Coagulation rapidly neutralizes charges on particles, leading to particle aggregation and improved sedimentation with relatively lower chemical doses, though it may produce fine precipitates that are harder to settle and can increase sludge volume. Your choice between flocculation and coagulation depends on water quality parameters, treatment goals, and operational stability to ensure efficient contaminant removal.
Conclusion: Choosing Between Flocculation and Coagulation
Choosing between flocculation and coagulation depends on the specific water treatment goals and the nature of suspended particles. Coagulation is effective for neutralizing particle charges and destabilizing colloids, while flocculation promotes the aggregation of these destabilized particles into larger flocs for easier removal. Optimal treatment often involves a combination of both processes to ensure maximum contaminant removal and water clarity.
flocculation vs coagulation Infographic
 libmatt.com
libmatt.com