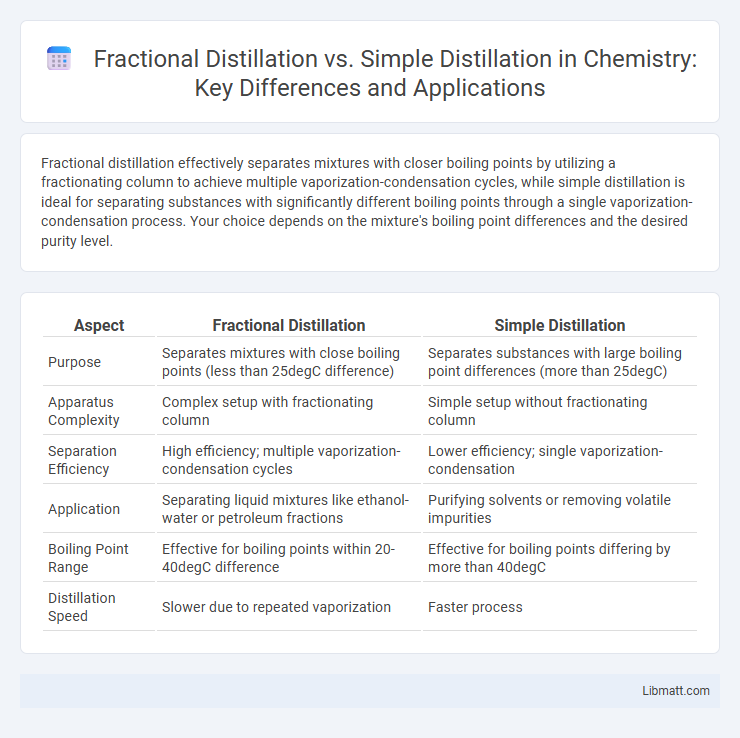
Fractional distillation effectively separates mixtures with closer boiling points by utilizing a fractionating column to achieve multiple vaporization-condensation cycles, while simple distillation is ideal for separating substances with significantly different boiling points through a single vaporization-condensation process. Your choice depends on the mixture's boiling point differences and the desired purity level.
Table of Comparison
| Aspect | Fractional Distillation | Simple Distillation |
|---|---|---|
| Purpose | Separates mixtures with close boiling points (less than 25degC difference) | Separates substances with large boiling point differences (more than 25degC) |
| Apparatus Complexity | Complex setup with fractionating column | Simple setup without fractionating column |
| Separation Efficiency | High efficiency; multiple vaporization-condensation cycles | Lower efficiency; single vaporization-condensation |
| Application | Separating liquid mixtures like ethanol-water or petroleum fractions | Purifying solvents or removing volatile impurities |
| Boiling Point Range | Effective for boiling points within 20-40degC difference | Effective for boiling points differing by more than 40degC |
| Distillation Speed | Slower due to repeated vaporization | Faster process |
Introduction to Distillation Methods
Simple distillation separates liquids with significantly different boiling points by heating a mixture until one component vaporizes and condenses, ideal for purifying solvents or separating liquids with at least a 25-30degC boiling point difference. Fractional distillation utilizes a fractionating column to separate mixtures with closer boiling points by providing repeated vaporization-condensation cycles, enabling more precise separation of complex mixtures like crude oil fractions. You should choose fractional distillation when dealing with compounds having boiling points less than 25degC apart to achieve higher purity and better separation efficiency.
What is Simple Distillation?
Simple distillation is a separation technique used to isolate a liquid from a mixture based on differing boiling points, typically effective when the components have a large boiling point difference. It involves boiling the liquid mixture, vaporizing the component with the lower boiling point, and then condensing the vapor back into liquid form. This method is ideal for purifying solvents or separating liquids from non-volatile impurities but is less efficient for separating liquids with similar boiling points compared to fractional distillation.
What is Fractional Distillation?
Fractional distillation is a technique used to separate mixtures of liquids with close boiling points by using a fractionating column that creates multiple vaporization-condensation cycles, enhancing the separation efficiency. Your distillation process benefits from fractional distillation when high purity is required, particularly in separating components of petroleum, alcohol, or chemical solvents. Unlike simple distillation, which is suitable for mixtures with large boiling point differences, fractional distillation provides precise separation for complex mixtures.
Key Principles Behind Each Technique
Simple distillation relies on the difference in boiling points between two substances, allowing separation when the boiling points differ by at least 25-30degC. Fractional distillation employs a fractionating column to create multiple condensation and vaporization cycles, enabling the separation of mixtures with closer boiling points, often less than 25degC apart. The key principle of fractional distillation is enhanced separation efficiency through repeated vapor-liquid equilibrium stages, unlike the single vaporization-condensation cycle in simple distillation.
Equipment Differences: Simple vs Fractional Distillation
Simple distillation uses a basic apparatus consisting of a distillation flask, condenser, and receiving flask, suitable for separating liquids with significantly different boiling points. Fractional distillation employs additional equipment like a fractionating column packed with glass beads or plates, enhancing separation efficiency by providing a larger surface area for repeated vaporization-condensation cycles. Understanding these equipment differences helps you choose the appropriate method for purifying mixtures with closer boiling points.
Applications of Simple Distillation
Simple distillation is primarily used to separate liquids with significantly different boiling points, such as purifying water from salt solutions or separating volatile solvents from non-volatile solutes. It is ideal for laboratory procedures involving the removal of low boiling impurities or collecting distilled water in chemical experiments. Industrial applications include producing distilled beverages and obtaining essential oils where components have large boiling point differences.
Applications of Fractional Distillation
Fractional distillation is widely used in industries such as petroleum refining, where it separates complex mixtures like crude oil into components like gasoline, diesel, and kerosene based on boiling points. This method is essential in chemical manufacturing for purifying solvents and producing high-purity liquids, including alcohols and essential oils. Your processes benefit from fractional distillation when precise separation of closely boiling substances is required, making it superior to simple distillation in complex mixtures.
Advantages and Limitations: Side-by-Side Comparison
Fractional distillation offers greater separation efficiency by using a fractionating column to achieve multiple vaporization-condensation cycles, making it ideal for mixtures with close boiling points. Simple distillation is faster and requires less equipment, but it is limited by lower purity of the collected fractions and is suitable primarily for mixtures with widely different boiling points. While fractional distillation excels in industrial applications like petroleum refining, simple distillation remains preferred for laboratory-scale separations involving volatile and non-volatile components.
Choosing the Right Distillation Method
Choosing the right distillation method depends on the boiling point differences of the components in the mixture; simple distillation suits mixtures with large boiling point gaps, typically greater than 70degC, for effective separation. Fractional distillation is ideal for separating components with closer boiling points, using a fractionating column to achieve higher purity by repeated vaporization-condensation cycles. Consider the desired purity and equipment complexity: simple distillation is quicker and less costly, while fractional distillation provides better separation for complex mixtures.
Summary: Distillation Method Selection Guide
Fractional distillation is ideal for separating liquid mixtures with close boiling points due to its multiple vaporization-condensation cycles, while simple distillation suits mixtures with significantly different boiling points or for removing volatile impurities. Your choice depends on the complexity of the mixture and the desired purity level; fractional distillation offers higher precision but requires more time and specialized equipment. Understanding these distinctions ensures efficient separation and optimal use of laboratory resources.
Fractional distillation vs simple distillation Infographic
 libmatt.com
libmatt.com