Oxychlorination uses a combination of hydrochloric acid and oxygen to produce chlorinated compounds with higher selectivity and fewer by-products, making it more environmentally friendly than direct chlorination. Your choice between oxychlorination and direct chlorination depends on factors such as desired product purity, process efficiency, and operational safety.
Table of Comparison
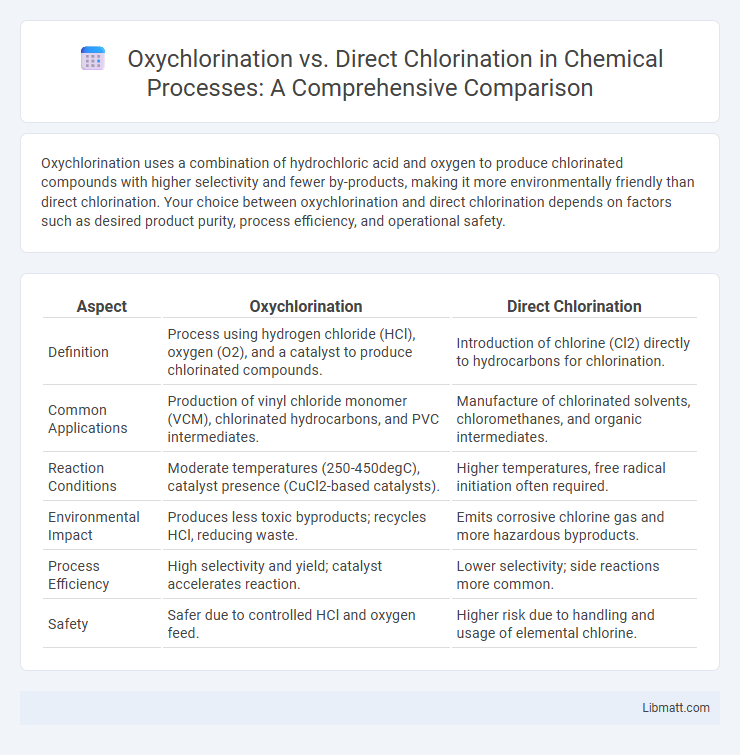
| Aspect | Oxychlorination | Direct Chlorination |
|---|---|---|
| Definition | Process using hydrogen chloride (HCl), oxygen (O2), and a catalyst to produce chlorinated compounds. | Introduction of chlorine (Cl2) directly to hydrocarbons for chlorination. |
| Common Applications | Production of vinyl chloride monomer (VCM), chlorinated hydrocarbons, and PVC intermediates. | Manufacture of chlorinated solvents, chloromethanes, and organic intermediates. |
| Reaction Conditions | Moderate temperatures (250-450degC), catalyst presence (CuCl2-based catalysts). | Higher temperatures, free radical initiation often required. |
| Environmental Impact | Produces less toxic byproducts; recycles HCl, reducing waste. | Emits corrosive chlorine gas and more hazardous byproducts. |
| Process Efficiency | High selectivity and yield; catalyst accelerates reaction. | Lower selectivity; side reactions more common. |
| Safety | Safer due to controlled HCl and oxygen feed. | Higher risk due to handling and usage of elemental chlorine. |
Introduction to Chlorination Methods
Chlorination methods include oxychlorination and direct chlorination, both essential in industrial chemical processes for producing chlorinated compounds. Oxychlorination involves the reaction of hydrocarbons with hydrogen chloride and oxygen over a catalyst, offering better control and fewer by-products compared to direct chlorination, which uses chlorine gas directly. Understanding these methods helps optimize your chemical production by selecting the most efficient and environmentally friendly process.
Overview of Oxychlorination
Oxychlorination is an industrial chemical process used primarily for producing vinyl chloride by reacting ethylene, hydrogen chloride, and oxygen over a copper-based catalyst. This method offers higher selectivity and lower environmental impact compared to direct chlorination, which involves reacting ethylene directly with chlorine gas and often generates more hazardous byproducts. The efficiency of oxychlorination lies in its ability to recycle hydrogen chloride within the process, reducing raw material consumption and improving overall yield.
Understanding Direct Chlorination
Direct chlorination involves the reaction of hydrocarbons with chlorine gas to produce chlorinated compounds, commonly used in the manufacture of solvents and intermediates. This method offers simplicity and cost-effectiveness but may lead to less selective products and higher formation of unwanted by-products compared to oxychlorination. Understanding direct chlorination helps optimize your chemical processes by balancing reactivity, selectivity, and operational conditions to achieve desired chlorinated products efficiently.
Key Chemical Reactions in Each Process
Oxychlorination involves the reaction of ethylene, hydrogen chloride, and oxygen over a catalyst to produce ethylene dichloride and water, represented by the reaction: C2H4 + 2HCl + 0.5O2 - C2H4Cl2 + H2O. Direct chlorination, on the other hand, entails the reaction of ethylene with chlorine to form ethylene dichloride without the involvement of oxygen, following the reaction: C2H4 + Cl2 - C2H4Cl2. The oxychlorination process offers better control over exothermic heat and results in fewer byproducts compared to direct chlorination.
Comparative Process Flow Diagrams
The comparative process flow diagrams of oxychlorination and direct chlorination highlight distinct operational sequences and feedstock requirements. Oxychlorination involves reacting methane with hydrogen chloride and oxygen over a catalyst to produce chloromethanes, with key steps including gas-phase reaction, quenching, and separation units. Direct chlorination features methane reacting directly with chlorine gas, facilitated by radical initiation and termination phases; its flow diagram emphasizes controlled chlorine injection, photolytic initiation, and byproduct handling equipment.
Catalyst Requirements and Differences
Oxychlorination requires copper-based catalysts, typically copper(II) chloride supported on alumina, to facilitate the oxidation of hydrocarbons in the presence of HCl and oxygen, enhancing selectivity and yield. Direct chlorination operates without catalysts, relying on radical chain reactions initiated by ultraviolet light or heat, which often leads to lower selectivity and higher by-product formation. The catalytic process in oxychlorination allows for better control over chlorination degree and minimizes hazardous chlorine consumption compared to non-catalytic direct chlorination.
Product Yield and Purity Comparison
Oxychlorination typically achieves higher product yield and purity compared to direct chlorination due to its controlled reaction conditions and continuous regeneration of hydrochloric acid, which minimizes side reactions. Direct chlorination often results in lower selectivity and increased by-product formation, reducing overall yield and complicating purification processes. Industrial applications favor oxychlorination for producing high-purity chlorinated compounds with optimized efficiency.
Environmental Impact and By-product Management
Oxychlorination generates fewer hazardous by-products compared to direct chlorination, reducing the release of toxic substances like dioxins and carcinogenic chlorinated organics into the environment. The process operates at lower temperatures, minimizing energy consumption and associated greenhouse gas emissions, while producing hydrogen chloride as a manageable by-product. In contrast, direct chlorination often leads to higher quantities of chlorinated waste requiring complex treatment, posing significant environmental disposal challenges.
Industrial Applications and Case Studies
Oxychlorination is widely used in the production of vinyl chloride monomer (VCM) for PVC manufacturing due to its cost-effectiveness and reduced corrosiveness compared to direct chlorination, which is favored for synthesizing chlorinated solvents and intermediates with higher purity requirements. Industrial case studies reveal that oxychlorination processes in facilities like those operated by Dow Chemical demonstrate improved energy efficiency and extended reactor lifespan, while direct chlorination is preferred in specialty chemical plants where precise control over chlorination levels is critical. Both methods are integral in large-scale chlorination operations, with oxychlorination catering to bulk chemical synthesis and direct chlorination supporting niche, high-purity applications.
Economic Considerations and Process Selection
Oxychlorination offers significant economic advantages over direct chlorination due to lower operational costs and reduced chlorine consumption, making it ideal for large-scale industrial applications. The process selection hinges on factors such as feedstock availability, desired product quality, and energy efficiency, with oxychlorination favored when minimizing hazardous byproducts and maximizing yield are priorities. Your choice should weigh capital investment, maintenance expenses, and environmental compliance to optimize overall profitability and sustainability.
Oxychlorination vs direct chlorination Infographic
 libmatt.com
libmatt.com