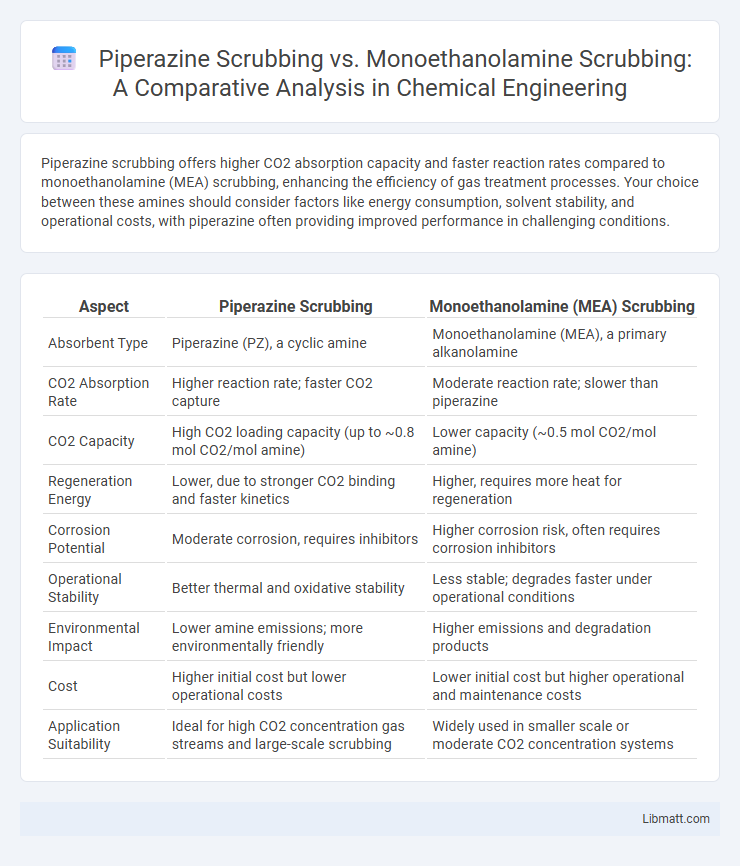
Piperazine scrubbing offers higher CO2 absorption capacity and faster reaction rates compared to monoethanolamine (MEA) scrubbing, enhancing the efficiency of gas treatment processes. Your choice between these amines should consider factors like energy consumption, solvent stability, and operational costs, with piperazine often providing improved performance in challenging conditions.
Table of Comparison
| Aspect | Piperazine Scrubbing | Monoethanolamine (MEA) Scrubbing |
|---|---|---|
| Absorbent Type | Piperazine (PZ), a cyclic amine | Monoethanolamine (MEA), a primary alkanolamine |
| CO2 Absorption Rate | Higher reaction rate; faster CO2 capture | Moderate reaction rate; slower than piperazine |
| CO2 Capacity | High CO2 loading capacity (up to ~0.8 mol CO2/mol amine) | Lower capacity (~0.5 mol CO2/mol amine) |
| Regeneration Energy | Lower, due to stronger CO2 binding and faster kinetics | Higher, requires more heat for regeneration |
| Corrosion Potential | Moderate corrosion, requires inhibitors | Higher corrosion risk, often requires corrosion inhibitors |
| Operational Stability | Better thermal and oxidative stability | Less stable; degrades faster under operational conditions |
| Environmental Impact | Lower amine emissions; more environmentally friendly | Higher emissions and degradation products |
| Cost | Higher initial cost but lower operational costs | Lower initial cost but higher operational and maintenance costs |
| Application Suitability | Ideal for high CO2 concentration gas streams and large-scale scrubbing | Widely used in smaller scale or moderate CO2 concentration systems |
Introduction to CO2 Scrubbing Technologies
CO2 scrubbing technologies primarily use chemical solvents like Piperazine and Monoethanolamine (MEA) to capture carbon dioxide from gas streams. Piperazine offers faster reaction kinetics and higher capacity for CO2 absorption compared to MEA, resulting in improved efficiency in industrial gas treatment. MEA remains widely used due to its established technology base and operational familiarity despite higher energy consumption during regeneration.
Overview of Piperazine Scrubbing
Piperazine scrubbing utilizes piperazine as an activator in amine-based CO2 capture processes, enhancing the absorption rate of carbon dioxide compared to conventional monoethanolamine (MEA) solutions. This method offers higher CO2 loading capacities and improved cyclic capacity, resulting in lower energy consumption during solvent regeneration. Industrial applications of piperazine scrubbing demonstrate increased operational stability and reduced corrosion concerns relative to MEA-based systems.
Principles of Monoethanolamine (MEA) Scrubbing
Monoethanolamine (MEA) scrubbing operates on the principle of chemical absorption, where MEA reacts with acidic gases like CO2 and H2S to form stable carbamate and ammonium salts. The process involves a reversible reaction allowing regeneration of MEA by heating, which releases the absorbed gases for capture or disposal. MEA scrubbing is characterized by high reaction rates and moderate energy consumption, making it effective for gas purification in industrial applications.
Thermodynamics and Reaction Mechanisms
Piperazine scrubbing exhibits faster reaction kinetics with CO2 due to its strong nucleophilicity and multiple amine groups, enhancing carbamate formation and increasing absorption rates compared to monoethanolamine (MEA). Thermodynamically, piperazine solutions maintain higher CO2 loading capacities and lower regeneration energy requirements, attributed to the stability of piperazine-CO2 complexes and favorable heat of reaction values. The reaction mechanism of piperazine involves the formation of stable zwitterions and bicarbonates, while MEA primarily undergoes a single-step carbamate formation, resulting in lower absorption efficiency and higher degradation rates under similar operating conditions.
Energy Consumption and Efficiency Comparison
Piperazine scrubbing demonstrates lower energy consumption compared to monoethanolamine (MEA) scrubbing due to its higher reaction rate and improved CO2 absorption capacity. The enhanced thermal stability of piperazine reduces solvent degradation, leading to lower regeneration energy requirements and increased operational efficiency. While MEA scrubbing remains widely used, piperazine-based systems offer significant energy savings and better overall carbon capture performance in industrial applications.
Corrosion and Equipment Longevity
Piperazine scrubbing offers enhanced corrosion resistance compared to monoethanolamine (MEA) scrubbing due to its higher thermal stability and lower oxidative degradation, which significantly reduces equipment wear and maintenance frequency. Your gas treating operations can benefit from extended equipment longevity and reduced downtime when using piperazine, as it minimizes the formation of corrosive by-products that typically accelerate metal corrosion. This results in improved operational reliability and cost savings over the lifecycle of gas scrubbing units.
Environmental Impacts and Byproduct Formation
Piperazine scrubbing produces lower levels of hazardous byproducts such as nitrosamines and nitramines compared to monoethanolamine (MEA) scrubbing, reducing environmental toxicity. MEA scrubbing generates significant ammonia emissions and high degradation byproduct formation, leading to greater air and water pollution concerns. The higher thermal stability of piperazine also results in less solvent degradation, minimizing secondary waste and environmental impact.
Operational Costs and Economic Considerations
Piperazine scrubbing generally offers lower operational costs compared to monoethanolamine (MEA) scrubbing due to its higher absorption capacity and faster reaction rates, which reduce solvent circulation rates and energy consumption. Economic considerations favor piperazine in large-scale applications where energy savings and reduced solvent degradation translate into significant cost reductions over time. Your choice between these amine scrubbing technologies should balance initial investment with long-term operational savings and maintenance requirements.
Industrial Applications and Case Studies
Piperazine scrubbing offers enhanced CO2 capture efficiency and increased absorption rates compared to monoethanolamine (MEA) scrubbing, making it favorable in large-scale natural gas processing and refinery emissions control. Industrial case studies highlight piperazine's superior thermal stability and lower energy requirements for solvent regeneration, improving operational costs and reducing environmental impact. MEA scrubbing remains widely used due to its lower initial cost and extensive performance data but faces challenges like solvent degradation and higher corrosion rates in continuous industrial applications.
Future Trends in Amine-Based CO2 Capture
Future trends in amine-based CO2 capture emphasize enhanced solvent formulations and process intensification to improve energy efficiency and reduce operational costs. Piperazine scrubbing exhibits higher CO2 absorption rates and thermal stability compared to monoethanolamine, making it a promising candidate for integration with advanced capture technologies such as membrane contactors and hybrid adsorption systems. Research prioritizes optimizing solvent blends and developing low-regeneration energy requirements to meet stringent carbon capture targets and support large-scale deployment.
Piperazine scrubbing vs monoethanolamine scrubbing Infographic
 libmatt.com
libmatt.com