Vacuum distillation separates components at lower pressures, reducing boiling points to protect heat-sensitive materials, while atmospheric distillation operates at standard pressure but requires higher temperatures. Your choice depends on the thermal stability of the substances and energy efficiency needs in the distillation process.
Table of Comparison
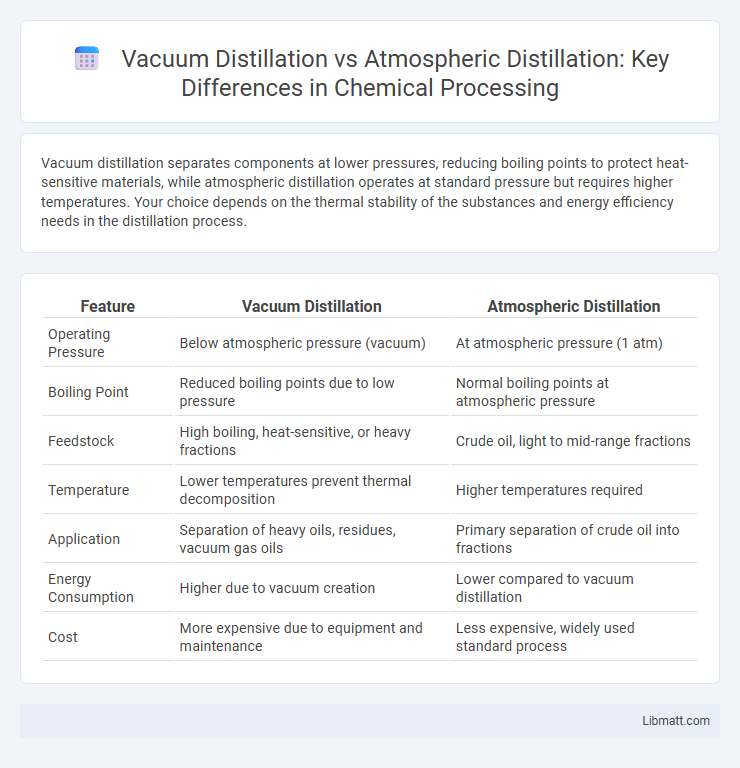
| Feature | Vacuum Distillation | Atmospheric Distillation |
|---|---|---|
| Operating Pressure | Below atmospheric pressure (vacuum) | At atmospheric pressure (1 atm) |
| Boiling Point | Reduced boiling points due to low pressure | Normal boiling points at atmospheric pressure |
| Feedstock | High boiling, heat-sensitive, or heavy fractions | Crude oil, light to mid-range fractions |
| Temperature | Lower temperatures prevent thermal decomposition | Higher temperatures required |
| Application | Separation of heavy oils, residues, vacuum gas oils | Primary separation of crude oil into fractions |
| Energy Consumption | Higher due to vacuum creation | Lower compared to vacuum distillation |
| Cost | More expensive due to equipment and maintenance | Less expensive, widely used standard process |
Introduction to Distillation Techniques
Vacuum distillation operates under reduced pressure, allowing the separation of components at lower temperatures, which prevents thermal decomposition of heat-sensitive substances, contrasting atmospheric distillation that functions at standard pressure and is suitable for components with higher boiling points. This technique enhances the efficiency of refining processes in petrochemical industries and is essential in producing purer fractions from complex mixtures. Understanding the differences between vacuum and atmospheric distillation helps optimize your choice depending on the thermal stability and boiling point of the materials involved.
What is Atmospheric Distillation?
Atmospheric distillation is a primary refining process that separates crude oil into various fractions based on boiling points at standard atmospheric pressure. This method involves heating crude oil to vaporize its components, allowing separation into gases, gasoline, kerosene, diesel, and heavier residues. Your refinery's efficiency depends on optimizing atmospheric distillation to maximize product yield before further processing like vacuum distillation.
What is Vacuum Distillation?
Vacuum distillation is a separation process that operates under reduced pressure, allowing components to boil at lower temperatures than their atmospheric boiling points. This technique is essential for purifying heat-sensitive materials or separating high-boiling-point compounds without thermal decomposition. Industries such as petrochemical refining and pharmaceutical manufacturing widely use vacuum distillation to enhance efficiency and product quality.
Key Differences Between Vacuum and Atmospheric Distillation
Vacuum distillation operates at pressures below atmospheric levels, enabling the separation of high-boiling-point substances at lower temperatures, which minimizes thermal degradation. Atmospheric distillation occurs at standard atmospheric pressure and is suitable for separating components with significantly different boiling points, commonly used in crude oil refining. The key difference lies in pressure conditions and temperature requirements, influencing energy consumption, equipment design, and the range of processable materials.
Applications of Atmospheric Distillation
Atmospheric distillation is widely utilized in petroleum refining to separate crude oil into essential fractions such as gasoline, diesel, kerosene, and lubricating oils. This process operates at atmospheric pressure, enabling the efficient separation of components with higher boiling points and making it suitable for large-scale industrial applications. Understanding how atmospheric distillation fits into your refinery operations can optimize product yield and quality.
Applications of Vacuum Distillation
Vacuum distillation is primarily used in the refining of heavy crude oils and the production of high-quality lubricants, where atmospheric distillation cannot effectively separate components due to high boiling points. This method lowers the boiling temperature of substances by reducing the pressure, preventing thermal decomposition of heat-sensitive compounds. Your refinery benefits from vacuum distillation by enabling the recovery of valuable heavy fractions that are essential for producing asphalt, waxes, and feedstock for catalytic cracking units.
Advantages of Vacuum Distillation
Vacuum distillation operates at lower pressures than atmospheric distillation, allowing the separation of high-boiling-point components at reduced temperatures, which prevents thermal decomposition and preserves product quality. This technique improves the yield of valuable heavy fractions, such as lubricating oils and waxes, by minimizing cracking and unwanted chemical changes. You benefit from enhanced energy efficiency and the ability to process heat-sensitive materials that atmospheric distillation cannot effectively handle.
Limitations of Atmospheric Distillation
Atmospheric distillation faces limitations such as lower boiling point ranges, increasing the risk of thermal decomposition for heat-sensitive compounds, and reduced efficiency in separating heavy fractions due to higher operating pressures. Your refining process may encounter increased energy consumption and reduced product yield when processing heavy crude oils under atmospheric conditions. Vacuum distillation overcomes these issues by lowering pressure to facilitate separation at reduced temperatures, improving overall efficiency and product quality.
Factors Influencing Distillation Method Choice
Vacuum distillation is preferred for heat-sensitive materials or compounds with high boiling points, as it reduces the boiling temperature by lowering the pressure, preventing thermal decomposition. Atmospheric distillation is suitable for substances stable at higher temperatures and occurs at standard atmospheric pressure, making it more energy-intensive but simpler and cost-effective for common mixtures. The choice between these methods depends on factors such as feed composition, thermal sensitivity, desired purity, and energy consumption.
Conclusion: Selecting the Right Distillation Process
Vacuum distillation operates at lower pressures, enabling the separation of high-boiling or heat-sensitive components without thermal decomposition, unlike atmospheric distillation which functions at standard pressure but is limited by higher boiling points. Your choice between vacuum and atmospheric distillation should consider the nature of the feedstock, thermal sensitivity, and desired product quality to optimize efficiency and yield. Selecting the right distillation process ensures effective separation while minimizing energy consumption and equipment wear.
Vacuum distillation vs atmospheric distillation Infographic
 libmatt.com
libmatt.com