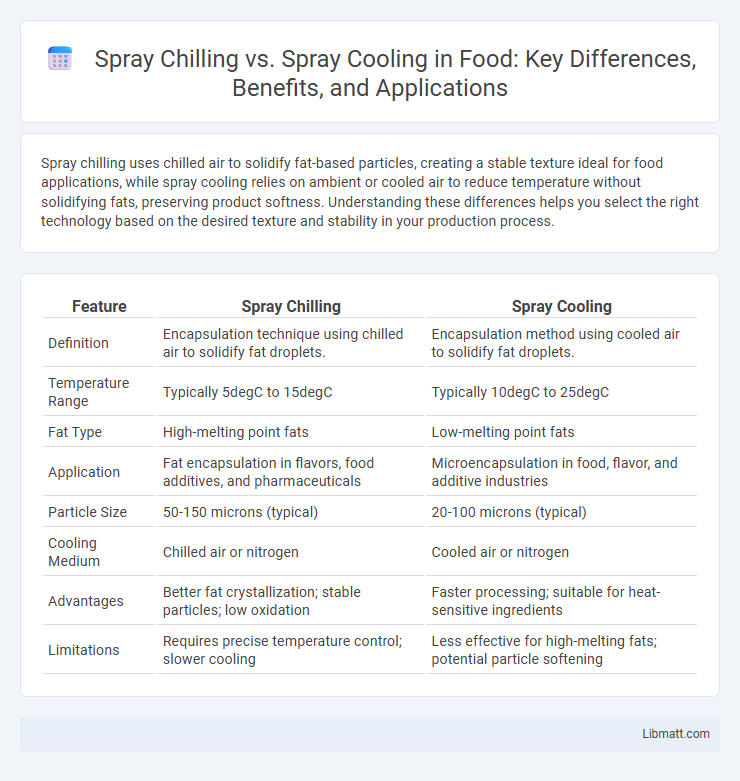
Spray chilling uses chilled air to solidify fat-based particles, creating a stable texture ideal for food applications, while spray cooling relies on ambient or cooled air to reduce temperature without solidifying fats, preserving product softness. Understanding these differences helps you select the right technology based on the desired texture and stability in your production process.
Table of Comparison
| Feature | Spray Chilling | Spray Cooling |
|---|---|---|
| Definition | Encapsulation technique using chilled air to solidify fat droplets. | Encapsulation method using cooled air to solidify fat droplets. |
| Temperature Range | Typically 5degC to 15degC | Typically 10degC to 25degC |
| Fat Type | High-melting point fats | Low-melting point fats |
| Application | Fat encapsulation in flavors, food additives, and pharmaceuticals | Microencapsulation in food, flavor, and additive industries |
| Particle Size | 50-150 microns (typical) | 20-100 microns (typical) |
| Cooling Medium | Chilled air or nitrogen | Cooled air or nitrogen |
| Advantages | Better fat crystallization; stable particles; low oxidation | Faster processing; suitable for heat-sensitive ingredients |
| Limitations | Requires precise temperature control; slower cooling | Less effective for high-melting fats; potential particle softening |
Introduction to Spray Chilling and Spray Cooling
Spray chilling involves atomizing molten materials into a cold air chamber, rapidly solidifying droplets into fine, free-flowing powders with improved stability and controlled particle size. Spray cooling, on the other hand, uses atomized liquid droplets exposed to a cooling environment that lowers temperature without necessarily changing the state of the material from liquid to solid. Both techniques play critical roles in food processing, pharmaceuticals, and chemical industries by enhancing the physical properties and shelf life of heat-sensitive substances.
How Spray Chilling Works
Spray chilling works by atomizing molten fat or lipid-based materials into fine droplets that are sprayed into a chilled chamber, causing rapid cooling and solidification upon contact with cold air. This process forms solid lipid particles with controlled size and texture, commonly used in pharmaceuticals and food industries to encapsulate sensitive ingredients or enhance product stability. The precise temperature control during spray chilling ensures uniform particle morphology and prevents degradation of heat-sensitive compounds.
How Spray Cooling Operates
Spray cooling operates by atomizing a chilled liquid onto a surface, where the liquid absorbs heat and evaporates, significantly lowering the surface temperature. This process efficiently removes heat through phase change, making it ideal for cooling electronic components and industrial equipment. The performance of spray cooling depends on factors such as droplet size, spray density, and evaporation rate, optimizing thermal management in high-heat environments.
Key Differences Between Spray Chilling and Spray Cooling
Spray chilling uses solid lipids that solidify upon cooling to encapsulate active ingredients, enhancing stability and controlled release, whereas spray cooling employs liquid lipids that solidify as they cool, often resulting in smoother particle formation. Spray chilling tends to produce particles with higher melting points, suited for temperature-sensitive applications, while spray cooling offers faster processing but may yield less thermal stability. The choice between spray chilling and spray cooling depends on desired particle characteristics, thermal properties, and application-specific requirements in pharmaceuticals or food industries.
Applications of Spray Chilling in Industry
Spray chilling finds extensive applications in the pharmaceutical industry for coating tablets and improving drug stability through controlled release formulations. The food industry employs spray chilling to encapsulate flavors, oils, and vitamins, enhancing shelf life and masking off-flavors. Additionally, the cosmetic sector uses spray chilling to produce solid lipid microparticles, improving the texture and delivery of active ingredients in skincare products.
Common Uses of Spray Cooling
Spray cooling is widely used in industrial processes such as metal forging, power plants, and electronics cooling due to its efficiency in heat dissipation and temperature regulation. It involves spraying a fine mist of water or coolant onto surfaces to maintain optimal operating temperatures and prevent overheating. This technique enhances equipment longevity and improves safety in high-heat environments.
Advantages of Spray Chilling
Spray chilling offers superior product stability by encapsulating active ingredients in a solid lipid matrix, protecting them from oxidation and moisture. This method enhances controlled release and improves the shelf life of sensitive compounds, making it ideal for pharmaceuticals and food applications. Your formulations benefit from reduced processing temperatures, preserving heat-sensitive nutrients and flavors more effectively than spray cooling.
Benefits of Spray Cooling
Spray cooling enhances product stability by rapidly solidifying ingredients, improving texture and shelf life in pharmaceutical and food industries. It provides efficient temperature control through the atomization of coolant sprays, reducing microbial growth and maintaining product integrity. This method also minimizes energy consumption compared to traditional cooling techniques, making it a sustainable choice for manufacturing processes.
Challenges and Limitations of Both Methods
Spray chilling faces challenges such as limited applicability with heat-sensitive materials due to the high cooling rates that may affect product quality, while spray cooling struggles with inefficiencies in energy consumption and slower cooling times. Both methods require precise control of particle size and temperature to avoid inconsistent texture and potential agglomeration, impacting the final product's performance. Understanding these limitations helps you optimize process parameters for better scalability and product consistency in food or pharmaceutical manufacturing.
Choosing the Right Method: Spray Chilling or Spray Cooling
Choosing between spray chilling and spray cooling depends on the formulation's thermal sensitivity and desired particle characteristics. Spray chilling solidifies molten lipids by cooling them below their melting point, ideal for heat-sensitive compounds requiring controlled release. Spray cooling involves rapid temperature reduction without phase change, suitable for encapsulating heat-stable ingredients and achieving uniform particle size distribution.
Spray Chilling vs Spray Cooling Infographic
 libmatt.com
libmatt.com