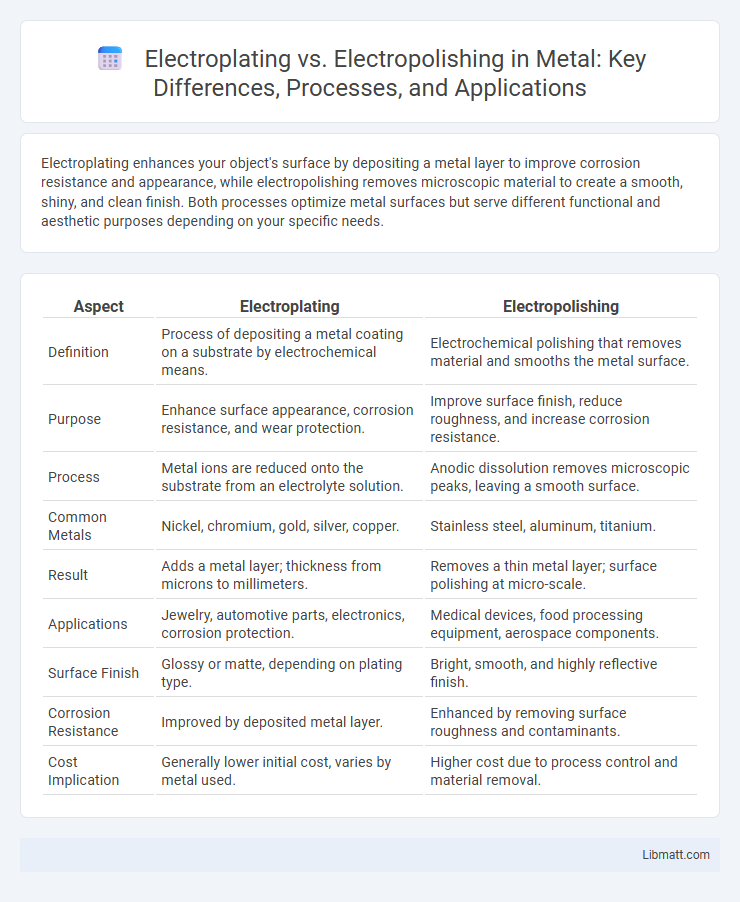
Electroplating enhances your object's surface by depositing a metal layer to improve corrosion resistance and appearance, while electropolishing removes microscopic material to create a smooth, shiny, and clean finish. Both processes optimize metal surfaces but serve different functional and aesthetic purposes depending on your specific needs.
Table of Comparison
| Aspect | Electroplating | Electropolishing |
|---|---|---|
| Definition | Process of depositing a metal coating on a substrate by electrochemical means. | Electrochemical polishing that removes material and smooths the metal surface. |
| Purpose | Enhance surface appearance, corrosion resistance, and wear protection. | Improve surface finish, reduce roughness, and increase corrosion resistance. |
| Process | Metal ions are reduced onto the substrate from an electrolyte solution. | Anodic dissolution removes microscopic peaks, leaving a smooth surface. |
| Common Metals | Nickel, chromium, gold, silver, copper. | Stainless steel, aluminum, titanium. |
| Result | Adds a metal layer; thickness from microns to millimeters. | Removes a thin metal layer; surface polishing at micro-scale. |
| Applications | Jewelry, automotive parts, electronics, corrosion protection. | Medical devices, food processing equipment, aerospace components. |
| Surface Finish | Glossy or matte, depending on plating type. | Bright, smooth, and highly reflective finish. |
| Corrosion Resistance | Improved by deposited metal layer. | Enhanced by removing surface roughness and contaminants. |
| Cost Implication | Generally lower initial cost, varies by metal used. | Higher cost due to process control and material removal. |
Introduction to Electroplating and Electropolishing
Electroplating is a surface finishing process that uses an electric current to deposit a thin layer of metal onto a substrate, enhancing corrosion resistance, appearance, and wear properties. Electropolishing is an electrochemical process that removes a thin layer of material from a metal surface to achieve a smooth, shiny, and contaminant-free finish. Both techniques rely on controlled electrical currents but serve different purposes: electroplating adds material, while electropolishing removes surface irregularities.
Defining Electroplating: Process and Applications
Electroplating is a process that uses electrical current to deposit a thin layer of metal onto the surface of a substrate, enhancing corrosion resistance, wear resistance, and aesthetic appeal. Common applications include coating jewelry, automotive parts, and electronic components to improve durability and conductivity. Understanding electroplating helps you select the right finish for your product's performance and visual requirements.
Defining Electropolishing: Process and Applications
Electropolishing is an electrochemical process that removes a thin layer of metal from a workpiece to improve surface finish, corrosion resistance, and cleanliness by creating a smooth and glossy finish. It is commonly applied to stainless steel, aluminum, and other metals in industries such as medical devices, aerospace, and food processing to enhance performance and aesthetics. Unlike electroplating, which deposits a metal coating, electropolishing selectively dissolves surface irregularities without adding material.
Key Differences Between Electroplating and Electropolishing
Electroplating deposits a thin layer of metal onto a substrate to enhance appearance, corrosion resistance, or conductivity, while electropolishing removes a thin layer of material from the surface to improve smoothness, brightness, and cleanliness. Electroplating uses metallic ions from a plating solution and applies electrical current to form the coating, whereas electropolishing employs anodic current to dissolve microscopic peaks on the metal surface, creating a polished finish. The key differences lie in electroplating adding material versus electropolishing removing material, along with their distinct purposes of coating enhancement versus surface refinement.
Surface Finish Comparison: Electroplating vs Electropolishing
Electroplating deposits a metal coating onto a substrate, often resulting in a uniform but sometimes rougher surface finish due to individual metal grain deposits. Electropolishing removes a microscopic layer of material, producing a smoother, brighter, and more reflective surface by selectively leveling microscopic peaks and valleys. The surface finish from electropolishing is generally superior in terms of smoothness and corrosion resistance compared to the thicker, sometimes textured coating achieved through electroplating.
Material Compatibility and Selection
Electroplating is compatible with a wide range of metals including steel, copper, and aluminum, allowing for surface enhancement through deposition of metals like nickel, chromium, or gold. Electropolishing is primarily used on stainless steel and other corrosion-resistant alloys to improve surface finish and remove micro-roughness without adding material. Selecting the appropriate process depends on the substrate material and the desired surface characteristics, with electroplating offering decorative and protective layers, while electropolishing optimizes cleanliness and corrosion resistance.
Industrial Uses: When to Choose Electroplating or Electropolishing
Electroplating is ideal for industries requiring corrosion resistance, enhanced surface conductivity, or decorative finishes, commonly used in automotive, electronics, and jewelry manufacturing. Electropolishing suits applications demanding improved surface smoothness, reduced microbial adhesion, and enhanced corrosion resistance, often employed in pharmaceutical equipment, food processing, and aerospace components. Choosing between electroplating and electropolishing depends on the need for surface coating versus surface refinement to optimize performance and durability in specific industrial environments.
Cost Considerations and Efficiency
Electroplating typically incurs higher costs due to material expenses and the need for frequent plating bath replenishment, while electropolishing offers cost savings by reducing surface roughness and enhancing corrosion resistance without additional material deposition. Electropolishing increases efficiency through uniform surface finishing and less waste production, whereas electroplating may require multiple layers and longer processing times to achieve desired thickness and quality. Choosing between the two depends on balancing upfront material costs against long-term maintenance and performance benefits.
Environmental and Safety Factors
Electroplating involves depositing metal layers using chemical baths that often contain hazardous substances like cyanides and heavy metals, posing environmental disposal challenges and requiring strict safety measures. Electropolishing, on the other hand, uses an electrochemical process to remove material from metal surfaces, producing fewer toxic byproducts and generally offering a safer, more environmentally friendly alternative. When choosing a finishing method, your priority should be minimizing harmful waste and ensuring workplace safety, making electropolishing advantageous in those areas.
Conclusion: Choosing the Right Surface Treatment
Electroplating involves depositing a metal coating to enhance corrosion resistance and aesthetic appeal, while electropolishing smooths and passivates the metal surface for improved cleanliness and reduced friction. Your choice depends on whether you prioritize protective layering or surface refinement, with electroplating suited for decorative and protective finishes, and electropolishing ideal for medical devices and semiconductor equipment requiring ultra-clean surfaces. Understanding the specific application requirements ensures optimal performance and longevity of your components.
Electroplating vs Electropolishing Infographic
 libmatt.com
libmatt.com