Metal hydrides store hydrogen by forming chemical bonds between metal atoms and hydrogen, offering high hydrogen capacity and fast kinetics, while intermetallic compounds consist of ordered alloys with distinct crystal structures, providing enhanced stability and reversible hydrogen absorption. Understanding these differences helps optimize your hydrogen storage solution based on capacity, kinetics, and cycling durability requirements.
Table of Comparison
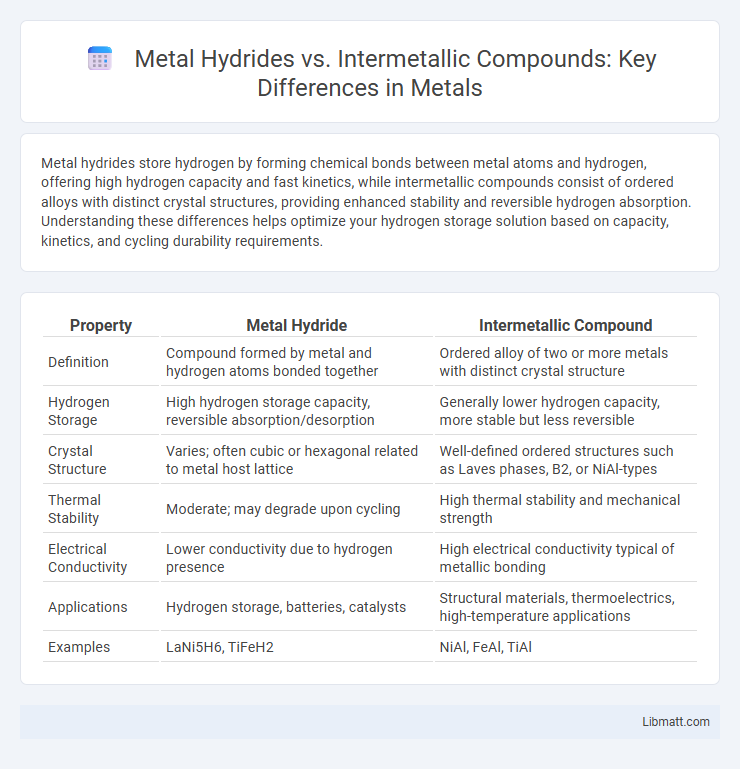
| Property | Metal Hydride | Intermetallic Compound |
|---|---|---|
| Definition | Compound formed by metal and hydrogen atoms bonded together | Ordered alloy of two or more metals with distinct crystal structure |
| Hydrogen Storage | High hydrogen storage capacity, reversible absorption/desorption | Generally lower hydrogen capacity, more stable but less reversible |
| Crystal Structure | Varies; often cubic or hexagonal related to metal host lattice | Well-defined ordered structures such as Laves phases, B2, or NiAl-types |
| Thermal Stability | Moderate; may degrade upon cycling | High thermal stability and mechanical strength |
| Electrical Conductivity | Lower conductivity due to hydrogen presence | High electrical conductivity typical of metallic bonding |
| Applications | Hydrogen storage, batteries, catalysts | Structural materials, thermoelectrics, high-temperature applications |
| Examples | LaNi5H6, TiFeH2 | NiAl, FeAl, TiAl |
Introduction to Metal Hydrides and Intermetallic Compounds
Metal hydrides are chemical compounds formed by the reaction of metals with hydrogen, often used for hydrogen storage due to their ability to absorb and release hydrogen efficiently. Intermetallic compounds consist of two or more metals with a distinct ordered atomic structure, offering unique physical and chemical properties different from their constituent metals. Understanding the differences between metal hydrides and intermetallic compounds is crucial for applications in energy storage, catalysis, and advanced material design.
Fundamental Definitions: Metal Hydrides vs Intermetallic Compounds
Metal hydrides are chemical compounds formed by the reaction of hydrogen with metals, where hydrogen atoms occupy interstitial sites in the metal lattice, enabling reversible hydrogen storage. Intermetallic compounds consist of two or more metals bonded in a fixed stoichiometric ratio with an ordered crystal structure, exhibiting distinct physical and chemical properties different from their constituent metals. While metal hydrides primarily focus on hydrogen-metal interactions for energy applications, intermetallic compounds are valued for their structural stability and functional properties in alloys and catalysis.
Structural Differences and Crystal Lattices
Metal hydrides typically form by the incorporation of hydrogen atoms into the interstitial sites of a metal's crystal lattice, often maintaining the host metal's original structure such as face-centered cubic (FCC) or hexagonal close-packed (HCP) lattices. Intermetallic compounds exhibit distinct, ordered crystal structures with fixed stoichiometries, characterized by well-defined atomic arrangements and specific lattice types like B2, L12, or CsCl-type structures. These structural differences influence hydrogen storage capacity and diffusion properties, as metal hydrides allow more flexible hydrogen accommodation while intermetallics provide stable, rigid frameworks with selective hydrogen absorption sites.
Chemical Bonding in Metal Hydrides and Intermetallics
Chemical bonding in metal hydrides involves the formation of ionic or covalent bonds between hydrogen atoms and metal atoms, resulting in hydrogen storage through metal-hydrogen interactions. Intermetallic compounds exhibit metallic bonding with a well-ordered atomic structure between two or more metals, influencing their electrical and mechanical properties. Understanding the distinct bonding mechanisms helps you tailor materials for applications such as hydrogen storage and high-strength alloys.
Synthesis Methods and Formation Mechanisms
Metal hydrides are typically synthesized via direct hydrogenation involving the exposure of pure metals or alloys to high-pressure hydrogen gas at elevated temperatures, promoting the diffusion of hydrogen atoms into the metal lattice. Intermetallic compounds form through solid-state reactions or melt solidification processes, often requiring precise stoichiometric control and annealing treatments to achieve ordered atomic arrangements. The formation mechanism of metal hydrides centers on the insertion of hydrogen into interstitial sites within the metal matrix, while intermetallic compounds result from diffusion-driven atomic rearrangements forming distinct crystal structures with specific compositional ratios.
Physical and Chemical Properties Comparison
Metal hydrides typically exhibit high hydrogen storage capacity and reversible hydrogen absorption due to their ability to form strong metal-hydrogen bonds, while intermetallic compounds often show enhanced structural stability and specific catalytic activity resulting from ordered atomic arrangements. Physically, metal hydrides can experience significant volume expansion during hydrogenation, impacting mechanical properties, whereas intermetallic compounds generally maintain more stable lattice parameters under similar conditions. Understanding these differences in hydrogen affinity and lattice dynamics is essential for optimizing Your material selection in hydrogen storage and energy applications.
Applications in Hydrogen Storage and Energy
Metal hydrides and intermetallic compounds both play crucial roles in hydrogen storage and energy applications due to their ability to absorb and release hydrogen efficiently. Metal hydrides offer high hydrogen storage density, making them ideal for fuel cell vehicles and portable energy systems, while intermetallic compounds provide excellent structural stability and faster kinetics for reversible hydrogen absorption, suitable for stationary energy storage and industrial uses. Your choice between the two depends on the required storage capacity, operating temperature, and application-specific energy efficiency.
Stability, Reactivity, and Environmental Impact
Metal hydrides generally exhibit high stability due to strong hydrogen-metal bonding, whereas intermetallic compounds often show varied stability influenced by their specific atomic arrangements. Reactivity in metal hydrides is typically higher, enabling efficient hydrogen storage and release, while intermetallic compounds tend to be less reactive but offer enhanced structural integrity. Environmentally, metal hydrides pose concerns related to hydrogen release and flammability, whereas intermetallic compounds are often more environmentally benign due to their inert behavior and reduced risk of hydrogen leakage.
Advantages and Limitations of Each Material Type
Metal hydrides offer high hydrogen storage capacity and reversible absorption-desorption cycles, making them ideal for energy storage and fuel cell applications. However, they often face limitations such as slow kinetics, high operating temperatures, and structural degradation over repeated cycles. Intermetallic compounds provide better structural stability and faster hydrogen absorption rates but typically have lower hydrogen storage capacity and can be more expensive to produce.
Future Perspectives and Research Trends
Future perspectives in metal hydride and intermetallic compound research emphasize enhancing hydrogen storage capacity, kinetics, and cycle stability for sustainable energy applications. Advanced computational modeling and nanostructuring techniques are driving innovations in material design to tailor atomic-scale interactions and optimize thermodynamic properties. Emerging research trends include developing lightweight, high-capacity alloys and exploring hybrid systems that combine metal hydrides with intermetallics to achieve improved performance in hydrogen storage and fuel cell technologies.
Metal hydride vs intermetallic compound Infographic
 libmatt.com
libmatt.com