Sintering involves heating powdered materials below their melting point to bond particles without liquefying, preserving structural integrity and reducing distortion. Melting requires raising the temperature above the melting point, causing materials to liquefy for molding or casting but often leading to significant dimensional changes.
Table of Comparison
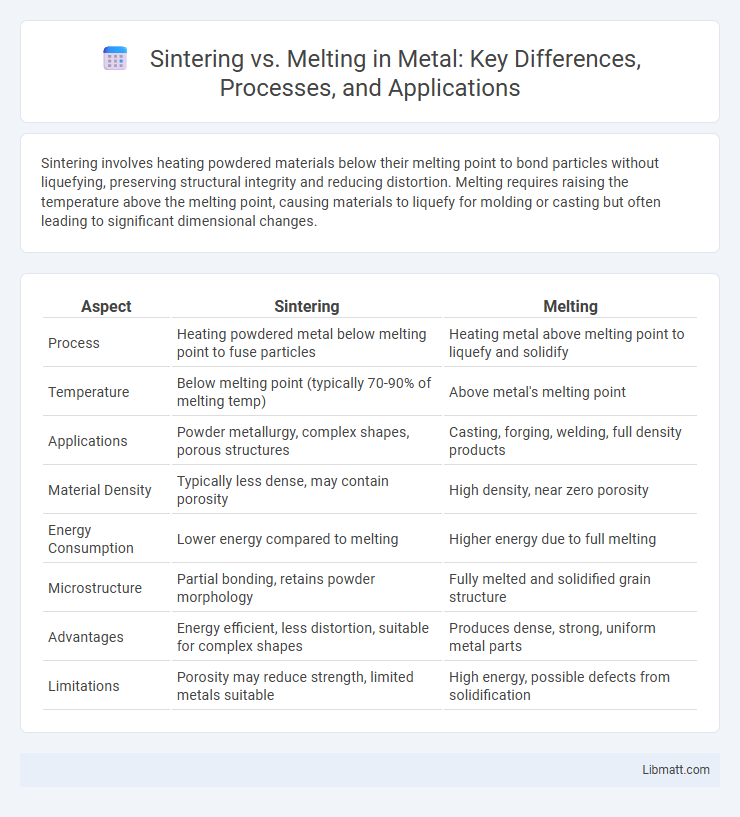
| Aspect | Sintering | Melting |
|---|---|---|
| Process | Heating powdered metal below melting point to fuse particles | Heating metal above melting point to liquefy and solidify |
| Temperature | Below melting point (typically 70-90% of melting temp) | Above metal's melting point |
| Applications | Powder metallurgy, complex shapes, porous structures | Casting, forging, welding, full density products |
| Material Density | Typically less dense, may contain porosity | High density, near zero porosity |
| Energy Consumption | Lower energy compared to melting | Higher energy due to full melting |
| Microstructure | Partial bonding, retains powder morphology | Fully melted and solidified grain structure |
| Advantages | Energy efficient, less distortion, suitable for complex shapes | Produces dense, strong, uniform metal parts |
| Limitations | Porosity may reduce strength, limited metals suitable | High energy, possible defects from solidification |
Introduction: Sintering vs Melting
Sintering involves heating powdered materials below their melting points to bond particles through diffusion, preserving the original solid structure while enhancing strength and density. Melting requires raising the material to a temperature above its melting point, causing it to transition into a liquid state for reshaping or casting. The choice between sintering and melting depends on factors like material type, desired mechanical properties, and production efficiency.
Defining Sintering: Process and Principles
Sintering is a manufacturing process that compacts and forms solid materials by heating powdered particles below their melting point until they bond through diffusion mechanisms. This technique relies on atomic diffusion to fuse particles at grain boundaries without reaching the liquid phase, preserving the material's microstructure and mechanical properties. Commonly used in powder metallurgy and ceramics, sintering enables precise control over density, porosity, and strength in the final product.
Understanding Melting: Mechanism and Applications
Melting is the process where a solid material transforms into a liquid upon reaching its melting point, driven by the absorption of heat energy that breaks atomic bonds. This mechanism is fundamental in applications such as metal casting, glass manufacturing, and additive manufacturing, where precise control over temperature and material properties is critical. Understanding melting enables you to optimize processes that require phase changes for shaping, joining, or refining materials with desired structural and functional characteristics.
Key Differences Between Sintering and Melting
Sintering involves heating powdered material below its melting point to bond particles through diffusion, preserving the original solid structure, while melting raises the material above its melting point to create a liquid phase that solidifies upon cooling. Sintering maintains porosity and dimensional stability, making it ideal for producing complex shapes without full liquefaction. Your choice between sintering and melting depends on the desired material properties, structural integrity, and manufacturing precision.
Temperature Requirements: Sintering vs Melting
Sintering typically occurs at temperatures between 70% and 90% of a material's melting point, allowing particles to bond without full liquefaction, which preserves the overall structure. Melting requires reaching or exceeding the exact melting temperature, transforming the solid into a liquid state. Sintering's lower temperature requirement results in less energy consumption and minimized thermal distortion compared to melting.
Material Properties After Sintering and Melting
Material properties after sintering typically include enhanced mechanical strength, improved hardness, and better wear resistance due to particle bonding without reaching the material's melting point, preserving grain structure. In contrast, melting leads to complete liquefaction, resulting in a homogeneous microstructure upon solidification, often with superior ductility but potential risks of porosity and grain growth. Comparing sintering versus melting reveals critical differences in density, microstructural integrity, and residual stresses that directly impact final product performance.
Industrial Applications of Sintering
Sintering is widely used in industrial applications such as the manufacturing of metal parts, ceramics, and composites, offering precise control over shape and mechanical properties without reaching the melting point. This process enhances material strength and density by bonding powdered particles through heat and pressure, making it ideal for producing complex components in automotive, aerospace, and electronics industries. Your production efficiency benefits from sintering's ability to reduce material waste and energy consumption compared to traditional melting methods.
Industrial Applications of Melting
Melting plays a critical role in industrial applications such as metal casting, where molten metals are poured into molds to produce complex shapes with high precision. It is essential in glass manufacturing, allowing raw materials to transform into uniform molten glass for shaping and forming. The process also supports alloy production, enabling consistent mixing of different metals to achieve desired mechanical properties.
Advantages and Disadvantages: Sintering vs Melting
Sintering offers advantages such as lower energy consumption, reduced material waste, and the ability to produce complex shapes with fine microstructures, though it may result in lower mechanical strength compared to melting. Melting allows for complete material fusion, leading to higher density and strength but typically requires higher temperatures and can cause undesirable grain growth or distortion. Choosing between sintering and melting depends on factors like material type, desired properties, and manufacturing costs.
Choosing the Right Process: Factors to Consider
Selecting between sintering and melting depends on material properties, desired structural integrity, and energy efficiency. Sintering offers precise control over porosity and lower energy consumption, ideal for ceramics and metal powders, while melting suits applications requiring full densification and homogenous microstructures. Your choice must weigh production volume, mechanical requirements, and thermal sensitivity to optimize performance and cost-effectiveness.
Sintering vs Melting Infographic
 libmatt.com
libmatt.com