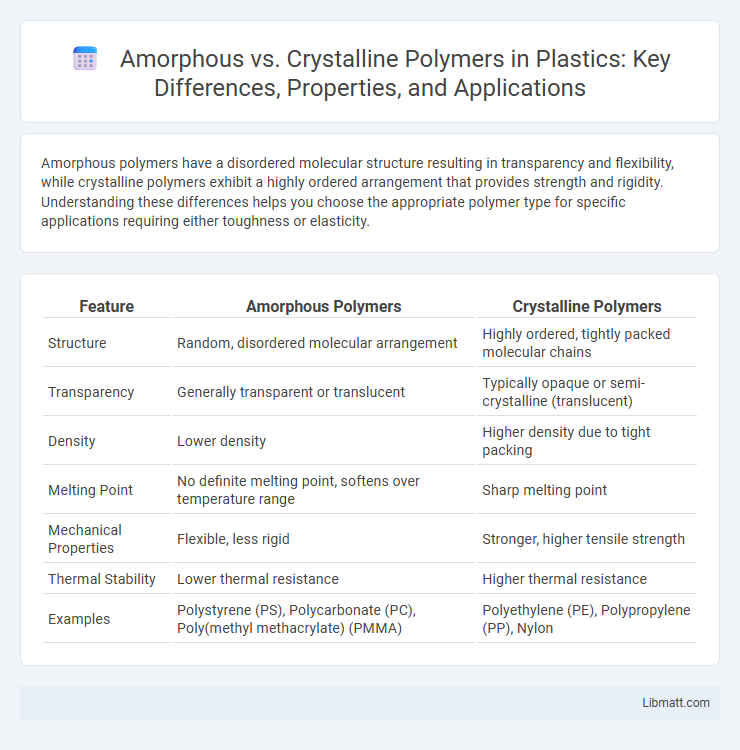
Amorphous polymers have a disordered molecular structure resulting in transparency and flexibility, while crystalline polymers exhibit a highly ordered arrangement that provides strength and rigidity. Understanding these differences helps you choose the appropriate polymer type for specific applications requiring either toughness or elasticity.
Table of Comparison
| Feature | Amorphous Polymers | Crystalline Polymers |
|---|---|---|
| Structure | Random, disordered molecular arrangement | Highly ordered, tightly packed molecular chains |
| Transparency | Generally transparent or translucent | Typically opaque or semi-crystalline (translucent) |
| Density | Lower density | Higher density due to tight packing |
| Melting Point | No definite melting point, softens over temperature range | Sharp melting point |
| Mechanical Properties | Flexible, less rigid | Stronger, higher tensile strength |
| Thermal Stability | Lower thermal resistance | Higher thermal resistance |
| Examples | Polystyrene (PS), Polycarbonate (PC), Poly(methyl methacrylate) (PMMA) | Polyethylene (PE), Polypropylene (PP), Nylon |
Introduction to Polymer Structures
Amorphous polymers exhibit a random, disordered molecular arrangement resulting in flexible and transparent materials, while crystalline polymers feature highly ordered, tightly packed chains that contribute to increased strength and thermal resistance. The degree of crystallinity directly influences polymer properties such as density, melting point, and mechanical performance. Understanding these structural differences helps you select the appropriate polymer type for specific applications requiring either flexibility or rigidity.
Definition of Amorphous Polymers
Amorphous polymers are materials characterized by a disordered molecular structure in which polymer chains are arranged randomly without a definite geometric pattern. This lack of crystalline regions results in unique properties such as transparency, flexibility, and lower density compared to crystalline polymers. Understanding the definition of amorphous polymers can help you select materials suited for applications requiring impact resistance and optical clarity.
Definition of Crystalline Polymers
Crystalline polymers are materials characterized by a highly ordered molecular structure where polymer chains are arranged in a regular, repeating pattern, resulting in regions known as crystallites. These polymers exhibit distinct melting points due to their structured lattice, which imparts enhanced mechanical strength, chemical resistance, and thermal stability compared to amorphous polymers. Common examples include polyethylene, polypropylene, and nylon, which benefit from their degree of crystallinity in applications requiring durability and rigidity.
Molecular Arrangement: Amorphous vs Crystalline
Amorphous polymers exhibit a random, disordered molecular arrangement lacking a defined geometric pattern, resulting in increased flexibility and transparency. Crystalline polymers have molecules arranged in a highly ordered, repeating lattice structure that enhances strength and thermal resistance. Understanding these distinct molecular arrangements helps you select the right polymer for applications requiring specific mechanical and optical properties.
Physical Properties Comparison
Amorphous polymers exhibit a random molecular arrangement, resulting in transparency, lower density, and improved impact resistance, whereas crystalline polymers possess highly ordered chains that enhance mechanical strength, chemical resistance, and thermal stability. The melting point of crystalline polymers is sharply defined, while amorphous polymers soften over a range of temperatures due to their glass transition behavior. Differences in crystallinity directly influence properties such as tensile strength, stiffness, and barrier resistance, making material selection critical for specific applications.
Thermal Behavior and Stability
Amorphous polymers exhibit a glass transition temperature (Tg) where they transition from a rigid to a rubbery state, while crystalline polymers show a distinct melting temperature (Tm) due to their ordered structure. The thermal stability of crystalline polymers is generally higher, attributed to strong intermolecular forces in their lattice, which enhance resistance to thermal degradation. Understanding these differences enables you to select polymers with suitable thermal properties for applications requiring heat resistance or flexibility.
Mechanical Properties and Performance
Amorphous polymers exhibit lower stiffness and higher impact resistance due to their random molecular arrangement, resulting in greater flexibility and toughness. Crystalline polymers have tightly packed chains with strong intermolecular forces, providing higher tensile strength, hardness, and dimensional stability under mechanical stress. Your choice between amorphous and crystalline polymers significantly influences performance factors such as durability, elasticity, and load-bearing capacity in various engineering applications.
Applications of Amorphous Polymers
Amorphous polymers, such as polystyrene and polycarbonate, are widely used in applications requiring optical clarity and impact resistance, including lenses, protective shields, and electronic displays. Their random molecular structure provides excellent transparency and good dimensional stability, making them ideal for medical devices and packaging materials. These polymers also exhibit good processability for injection molding and extrusion, supporting diverse manufacturing needs in automotive and consumer goods industries.
Applications of Crystalline Polymers
Crystalline polymers, characterized by their highly ordered molecular structure, are extensively used in applications requiring enhanced mechanical strength, chemical resistance, and thermal stability. Common uses include automotive parts, packaging films, and medical devices where durability and clarity are essential. Your choice of crystalline polymers ensures improved performance in demanding environments due to their superior crystallinity and structural integrity.
Choosing Between Amorphous and Crystalline Polymers
Choosing between amorphous and crystalline polymers depends on your application's need for transparency, mechanical strength, and thermal resistance. Amorphous polymers, with their random molecular structure, offer excellent clarity and impact resistance but lower chemical resistance and melting points. Crystalline polymers provide higher tensile strength, chemical resistance, and thermal stability due to their ordered molecular arrangement, making them ideal for demanding structural components.
Amorphous vs Crystalline Polymers Infographic
 libmatt.com
libmatt.com