Glass transition temperature (Tg) marks the point where an amorphous material like glass or polymers switch from a brittle, glassy state to a more flexible, rubbery state, while melting temperature (Tm) is where a crystalline solid transitions into a liquid. Understanding these temperatures is crucial for selecting materials that must endure specific thermal conditions without losing structural integrity or performance.
Table of Comparison
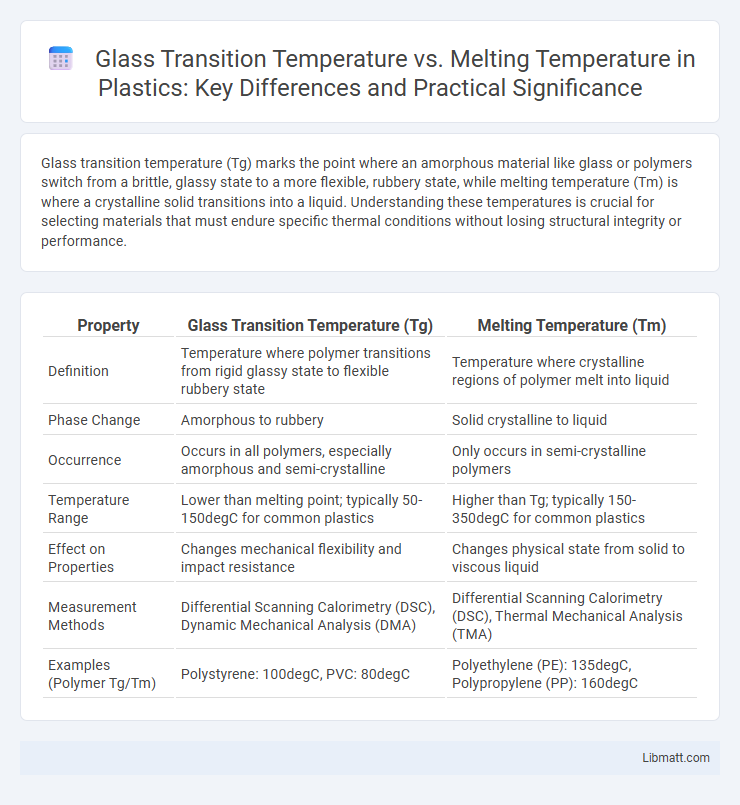
| Property | Glass Transition Temperature (Tg) | Melting Temperature (Tm) |
|---|---|---|
| Definition | Temperature where polymer transitions from rigid glassy state to flexible rubbery state | Temperature where crystalline regions of polymer melt into liquid |
| Phase Change | Amorphous to rubbery | Solid crystalline to liquid |
| Occurrence | Occurs in all polymers, especially amorphous and semi-crystalline | Only occurs in semi-crystalline polymers |
| Temperature Range | Lower than melting point; typically 50-150degC for common plastics | Higher than Tg; typically 150-350degC for common plastics |
| Effect on Properties | Changes mechanical flexibility and impact resistance | Changes physical state from solid to viscous liquid |
| Measurement Methods | Differential Scanning Calorimetry (DSC), Dynamic Mechanical Analysis (DMA) | Differential Scanning Calorimetry (DSC), Thermal Mechanical Analysis (TMA) |
| Examples (Polymer Tg/Tm) | Polystyrene: 100degC, PVC: 80degC | Polyethylene (PE): 135degC, Polypropylene (PP): 160degC |
Introduction to Glass Transition and Melting Temperatures
Glass Transition Temperature (Tg) marks the reversible change in amorphous materials from a hard and brittle state to a soft and rubbery state due to increased molecular mobility. Melting Temperature (Tm) is the specific heat energy point where crystalline materials transition from solid to liquid, indicating the breakdown of ordered molecular structures. Understanding Tg and Tm is essential for optimizing polymer processing, thermal stability, and material performance in various industrial applications.
Defining Glass Transition Temperature (Tg)
Glass Transition Temperature (Tg) marks the temperature at which an amorphous material, such as polymers or glasses, transitions from a hard, brittle state to a soft, rubbery state without melting. Unlike the Melting Temperature (Tm), which is a sharp, well-defined point where crystalline materials change from solid to liquid, Tg is a range reflecting molecular motion and flexibility within the material structure. Understanding Tg is crucial for applications requiring specific thermal and mechanical properties, as it influences material stability, durability, and performance under varying temperature conditions.
Understanding Melting Temperature (Tm)
Melting Temperature (Tm) represents the precise point at which a crystalline polymer transitions from a solid to a liquid state, characterized by the disruption of its ordered molecular structure. This temperature is critical for processing and application, as it defines the thermal stability and mechanical properties of polymers during heating. Unlike the Glass Transition Temperature (Tg), which involves a transition in amorphous regions, Tm specifically applies to crystalline segments, indicating a complete phase change.
Key Differences Between Tg and Tm
Glass Transition Temperature (Tg) marks the reversible transition of a polymer from a hard, glassy state to a soft, rubbery state without a phase change, whereas Melting Temperature (Tm) represents the exact point where a crystalline solid becomes a liquid. Tg is crucial for understanding polymer flexibility and thermal resistance below the melting point, while Tm defines the thermal limit for crystalline phase stability. Your material selection depends on whether flexibility or phase change behavior at elevated temperatures is more critical for the application.
Molecular Behavior at Glass Transition vs Melting Point
At the glass transition temperature (Tg), amorphous materials shift from a rigid, glassy state to a more rubbery, viscous state due to increased molecular mobility without breaking crystalline order, characterized by segmental motion of polymer chains. In contrast, at the melting temperature (Tm), crystalline materials undergo a phase change from solid to liquid, where ordered molecular structures break down completely, allowing for free molecular diffusion. The molecular behavior at Tg reflects cooperative segmental motion within a disordered matrix, while at Tm, it involves overcoming lattice energy to transition into a fluid phase.
Significance in Polymer Science
Glass Transition Temperature (Tg) marks the temperature at which a polymer transitions from a hard, glassy state to a soft, rubbery state, crucial for determining polymer flexibility and usability in various applications. Melting Temperature (Tm) indicates the point where crystalline regions of a polymer melt, reflecting its thermal stability and processing limits. Understanding these temperatures allows you to optimize polymer selection and processing conditions for desired mechanical and thermal performance.
Methods for Measuring Tg and Tm
Differential Scanning Calorimetry (DSC) is the primary method for measuring both the Glass Transition Temperature (Tg) and Melting Temperature (Tm), providing precise thermal analysis by detecting changes in heat flow. Dynamic Mechanical Analysis (DMA) is frequently used to assess Tg by monitoring changes in mechanical properties such as modulus and damping as temperature varies. Thermomechanical Analysis (TMA) can also measure Tg through dimensional changes while Tm is confirmed through visual observation during melting or by X-ray diffraction detecting crystal structure alterations.
Factors Influencing Glass Transition and Melting Temperatures
Glass transition temperature (Tg) and melting temperature (Tm) are influenced by polymer chain flexibility, molecular weight, and crystallinity. Higher chain rigidity and increased molecular weight typically raise Tg due to restricted segmental motion, while increased crystallinity elevates Tm by requiring more energy to break ordered structures. Your material's additives and processing conditions also play a critical role in modifying these thermal properties for specific applications.
Applications Requiring Tg and Tm Knowledge
Understanding the glass transition temperature (Tg) and melting temperature (Tm) is crucial in polymer manufacturing and material science for determining processing conditions and final product performance. Packaging industries rely on Tg data to ensure flexibility and durability at various temperatures, while Tm guides the melting and molding phases in thermoplastic production. Electronics and aerospace sectors utilize Tg to assess thermal stability and mechanical properties, while Tm is essential for selecting materials with appropriate crystallinity and heat resistance.
Summary: Choosing Materials Based on Tg and Tm
Glass transition temperature (Tg) and melting temperature (Tm) are critical thermal properties that influence material selection for specific applications. Tg indicates the temperature at which a polymer transitions from a hard, glassy state to a soft, rubbery state, affecting impact resistance and flexibility, while Tm defines the point where a crystalline material becomes liquid, determining thermal stability and processing limits. Understanding Your product's operating environment and mechanical requirements ensures optimal choice between materials with appropriate Tg and Tm for performance and durability.
Glass Transition Temperature vs Melting Temperature Infographic
 libmatt.com
libmatt.com