Peroxide resins cure through free radical polymerization initiated by organic peroxides, offering fast curing times and high crosslink density ideal for composites and coatings. Sulfur resins, formed by polymerizing elemental sulfur with unsaturated hydrocarbons, exhibit excellent thermal stability and chemical resistance, making them suitable for applications requiring durable, heat-resistant materials.
Table of Comparison
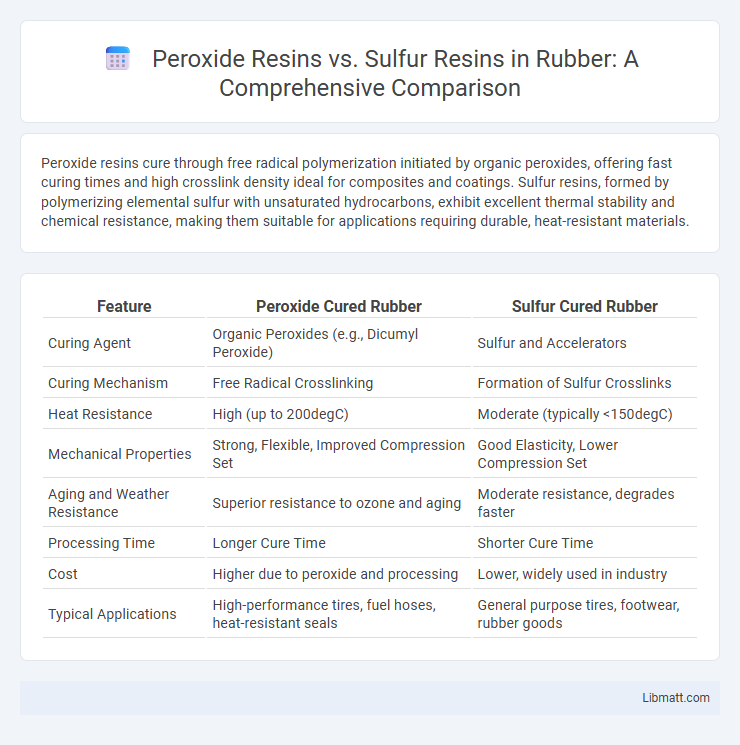
| Feature | Peroxide Cured Rubber | Sulfur Cured Rubber |
|---|---|---|
| Curing Agent | Organic Peroxides (e.g., Dicumyl Peroxide) | Sulfur and Accelerators |
| Curing Mechanism | Free Radical Crosslinking | Formation of Sulfur Crosslinks |
| Heat Resistance | High (up to 200degC) | Moderate (typically <150degC) |
| Mechanical Properties | Strong, Flexible, Improved Compression Set | Good Elasticity, Lower Compression Set |
| Aging and Weather Resistance | Superior resistance to ozone and aging | Moderate resistance, degrades faster |
| Processing Time | Longer Cure Time | Shorter Cure Time |
| Cost | Higher due to peroxide and processing | Lower, widely used in industry |
| Typical Applications | High-performance tires, fuel hoses, heat-resistant seals | General purpose tires, footwear, rubber goods |
Introduction to Peroxide Resins and Sulfur Resins
Peroxide resins are thermosetting polymers cured through free radical mechanisms initiated by peroxides, offering high heat resistance and chemical stability ideal for composites and coatings. Sulfur resins, derived from polymerizing elemental sulfur with unsaturated hydrocarbons, are valued for their adhesive properties, chemical resistance, and electrical insulation capabilities. Understanding the distinct curing processes and material properties of these resins helps you select the optimal resin type for applications demanding durability and specific performance characteristics.
Chemical Composition and Structure Overview
Peroxide resins are primarily composed of unsaturated polyester or vinyl monomers cross-linked through organic peroxide initiators, forming a rigid three-dimensional polymeric network. Sulfur resins consist mainly of polymerized elemental sulfur combined with aromatic hydrocarbons, resulting in a thermoplastic structure characterized by multiple sulfur-sulfur linkages. The distinct chemical compositions influence their thermal stability, mechanical properties, and curing mechanisms, with peroxide resins exhibiting high cross-link density and sulfur resins showcasing reversible sulfur chain structures.
Polymerization Mechanisms Compared
Peroxide resins undergo free-radical polymerization initiated by organic peroxides, generating reactive free radicals that facilitate chain growth and crosslinking in unsaturated monomers, resulting in highly crosslinked thermoset networks. Sulfur resins polymerize via a ring-opening mechanism where elemental sulfur forms polysulfide chains that crosslink with aromatic compounds through vulcanization-like processes, producing thermoplastics with enhanced thermal stability and chemical resistance. Your choice between peroxide and sulfur resins depends on the desired polymerization pathway, mechanical properties, and end-use thermal performance.
Key Physical Properties and Performance
Peroxide resins exhibit superior thermal stability and high tensile strength, making them ideal for high-performance composite applications where durability is critical. Sulfur resins offer excellent chemical resistance and lower viscosity, enhancing ease of processing and surface finish quality in coatings and adhesives. Your choice between peroxide and sulfur resins should depend on specific requirements for mechanical performance and environmental exposure.
Processing and Curing Methods
Peroxide resins cure through a free-radical polymerization mechanism initiated by organic peroxides, allowing for relatively fast curing at elevated temperatures typically between 120degC and 180degC. Sulfur resins undergo curing via sulfur cross-linking at moderate temperatures, generally in the range of 100degC to 150degC, which imparts improved thermal stability and solvent resistance but requires longer curing times. Processing peroxide resins benefits from rapid gelation and good control over cure kinetics, while sulfur resins demand precise temperature regulation to avoid premature cross-linking and ensure uniform network formation.
Applications in Industry
Peroxide resins are widely used in the automotive and packaging industries due to their excellent thermal stability and curing properties, making them ideal for composite materials and coatings. Sulfur resins find extensive applications in rubber vulcanization and oil refining processes, where their unique chemical resistance and durability enhance product performance. Your choice between peroxide and sulfur resins depends on the specific industrial requirements, such as thermal resistance or chemical compatibility.
Advantages of Peroxide Resins
Peroxide resins offer superior curing speed and enhanced thermal stability compared to sulfur resins, making them ideal for high-performance applications in industries such as automotive and aerospace. Their excellent cross-linking capability results in improved mechanical strength and chemical resistance, which ensures longer-lasting durability under harsh conditions. You benefit from faster production cycles and higher quality end products when opting for peroxide resins in your manufacturing processes.
Benefits of Sulfur Resins
Sulfur resins offer superior chemical resistance and thermal stability compared to peroxide resins, making them ideal for industrial coatings and adhesives exposed to harsh environments. Their low cost and ease of processing enhance production efficiency, while their excellent UV resistance ensures long-lasting performance in outdoor applications. Your projects can benefit from sulfur resins' exceptional durability and environmental resilience, providing reliable protection and extended material lifespan.
Environmental and Safety Considerations
Peroxide resins generate fewer hazardous emissions compared to sulfur resins, reducing environmental impact and improving workplace air quality. Sulfur resins often release sulfur-containing compounds that require rigorous ventilation and safety measures to protect workers and surrounding ecosystems. You should consider peroxide resins for safer handling and compliance with stricter environmental regulations in manufacturing processes.
Choosing Between Peroxide and Sulfur Resins: Key Factors
Choosing between peroxide resins and sulfur resins depends on factors such as curing speed, heat resistance, and mechanical properties. Peroxide resins offer faster curing and better heat resistance, making them suitable for high-performance composites, while sulfur resins provide excellent adhesion and flexibility for applications requiring impact resistance. Cost considerations and environmental impact also influence the selection, with sulfur resins often being more cost-effective and environmentally friendly.
Peroxide Resins vs Sulfur Resins Infographic
 libmatt.com
libmatt.com