Stearic acid is a saturated fatty acid commonly used as an emulsifier and thickening agent, while zinc stearate is a zinc salt of stearic acid often utilized as a lubricant and release agent in manufacturing processes. Understanding the differences between these compounds helps you select the appropriate ingredient for cosmetics, plastics, or rubber applications based on desired texture and performance.
Table of Comparison
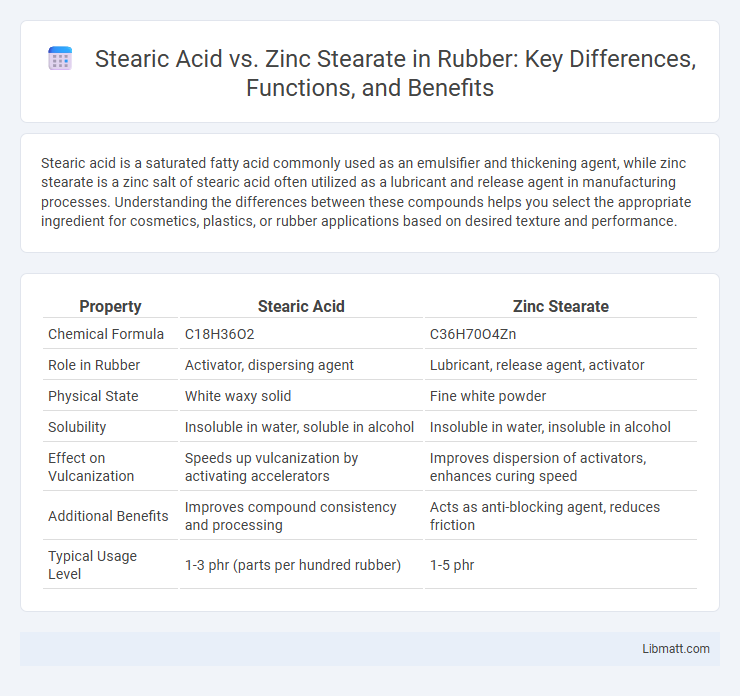
| Property | Stearic Acid | Zinc Stearate |
|---|---|---|
| Chemical Formula | C18H36O2 | C36H70O4Zn |
| Role in Rubber | Activator, dispersing agent | Lubricant, release agent, activator |
| Physical State | White waxy solid | Fine white powder |
| Solubility | Insoluble in water, soluble in alcohol | Insoluble in water, insoluble in alcohol |
| Effect on Vulcanization | Speeds up vulcanization by activating accelerators | Improves dispersion of activators, enhances curing speed |
| Additional Benefits | Improves compound consistency and processing | Acts as anti-blocking agent, reduces friction |
| Typical Usage Level | 1-3 phr (parts per hundred rubber) | 1-5 phr |
Introduction to Stearic Acid and Zinc Stearate
Stearic acid is a saturated fatty acid commonly derived from animal and plant fats, widely used in the production of cosmetics, soaps, and detergents due to its emollient and thickening properties. Zinc stearate, a compound formed by the reaction of stearic acid with zinc oxide, serves as a lubricant, release agent, and stabilizer in industries including plastics, rubber, and pharmaceuticals. Both substances are essential in manufacturing but differ significantly in chemical composition and applications.
Chemical Structure and Composition
Stearic acid is a saturated long-chain fatty acid with the chemical formula C18H36O2, characterized by a carboxyl group attached to a hydrocarbon chain. Zinc stearate consists of zinc ions coordinated with two stearate anions, forming the chemical formula Zn(C18H35O2)2, making it a metal salt derived from stearic acid. The fundamental difference lies in stearic acid being an organic acid molecule while zinc stearate is an inorganic compound combining zinc metal with stearate ions.
Sources and Production Methods
Stearic acid is primarily derived from animal fats and vegetable oils through hydrolysis or saponification processes, where triglycerides are broken down into fatty acids. Zinc stearate, on the other hand, is produced by neutralizing stearic acid with zinc oxide, forming a zinc salt commonly used as a lubricant and release agent. Understanding these distinct production methods helps you choose the appropriate compound based on your application requirements and source preferences.
Physical and Chemical Properties
Stearic acid is a saturated long-chain fatty acid with the molecular formula C18H36O2, exhibiting a waxy solid state at room temperature and a melting point around 69-70degC. Zinc stearate, a zinc salt of stearic acid with the formula Zn(C18H35O2)2, appears as a white, waxy powder that is insoluble in water and has a melting point approximately between 120-130degC. Chemically, stearic acid is acidic and participates in esterification reactions, whereas zinc stearate acts as a lubricant, water repellent, and stabilizer due to its hydrophobic and metal ion characteristics.
Industrial and Commercial Uses
Stearic Acid is widely used in the production of cosmetics, detergents, and lubricants due to its role as a surfactant and thickening agent. Zinc Stearate, known for its excellent water-repellent and anti-caking properties, is commonly utilized in the manufacture of rubber, plastics, and pharmaceuticals. Your choice between these compounds will depend on the specific industrial or commercial application requirements regarding texture, stability, and chemical resistance.
Performance in Formulations
Stearic acid offers excellent emollient and thickening properties, improving texture and stability in cosmetic and pharmaceutical formulations. Zinc stearate enhances mold release and lubrication, making it ideal for powder coatings, plastics, and rubber applications where smooth processing is critical. Your choice depends on whether the formulation requires conditioning benefits or improved mechanical performance and release characteristics.
Safety and Regulatory Considerations
Stearic acid and zinc stearate differ significantly in safety and regulatory profiles, with stearic acid generally recognized as safe (GRAS) by the FDA for food and cosmetic applications, exhibiting low toxicity and minimal skin irritation. Zinc stearate, while approved for industrial and cosmetic uses, is subject to stricter regulatory scrutiny due to potential respiratory hazards from inhalation of fine powders and its zinc content, necessitating adherence to occupational exposure limits set by OSHA and REACH regulations in Europe. Both compounds require proper handling and labeling to comply with global safety standards, especially in pharmaceutical, cosmetic, and food industries to ensure consumer protection and workplace safety.
Environmental Impact and Biodegradability
Stearic acid, derived primarily from vegetable oils, exhibits a favorable environmental impact due to its biodegradability and minimal aquatic toxicity, making it a sustainable choice in various industries. Zinc stearate, containing zinc ions, poses more ecological concerns as zinc can accumulate in soil and water, potentially disrupting ecosystems despite the stearate component's biodegradability. Your choice between these compounds should consider the balance between desired product performance and the environmental footprint, especially in applications prioritizing eco-friendly materials.
Cost and Availability Comparison
Stearic acid is generally more affordable and widely available due to its extensive use in cosmetics, food, and industrial applications. Zinc stearate, being a specialty compound primarily used as a lubricant and release agent in plastics and rubber industries, tends to be costlier and less readily available. Market demand and production complexity contribute significantly to the price and supply differences between these two stearate forms.
Choosing Between Stearic Acid and Zinc Stearate
Choosing between Stearic Acid and Zinc Stearate depends on your specific application in industries such as cosmetics, pharmaceuticals, or rubber manufacturing. Stearic Acid, a fatty acid, serves as an emulsifier and thickener, while Zinc Stearate acts primarily as a lubricant and release agent with anti-caking properties. Your selection should consider factors like solubility, compatibility with formulation ingredients, and desired functionality in the final product.
Stearic Acid vs Zinc Stearate Infographic
 libmatt.com
libmatt.com