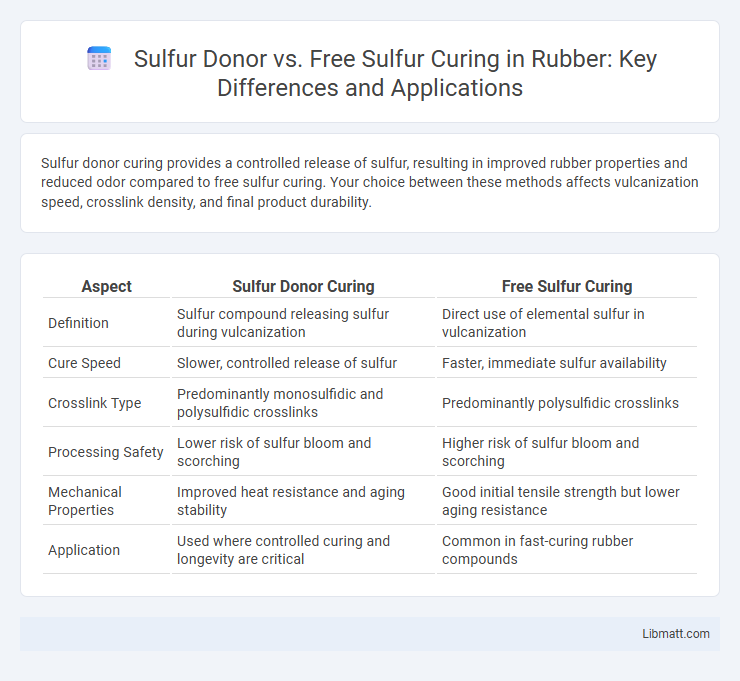
Sulfur donor curing provides a controlled release of sulfur, resulting in improved rubber properties and reduced odor compared to free sulfur curing. Your choice between these methods affects vulcanization speed, crosslink density, and final product durability.
Table of Comparison
| Aspect | Sulfur Donor Curing | Free Sulfur Curing |
|---|---|---|
| Definition | Sulfur compound releasing sulfur during vulcanization | Direct use of elemental sulfur in vulcanization |
| Cure Speed | Slower, controlled release of sulfur | Faster, immediate sulfur availability |
| Crosslink Type | Predominantly monosulfidic and polysulfidic crosslinks | Predominantly polysulfidic crosslinks |
| Processing Safety | Lower risk of sulfur bloom and scorching | Higher risk of sulfur bloom and scorching |
| Mechanical Properties | Improved heat resistance and aging stability | Good initial tensile strength but lower aging resistance |
| Application | Used where controlled curing and longevity are critical | Common in fast-curing rubber compounds |
Introduction to Sulfur Curing in Rubber
Sulfur curing in rubber involves the formation of cross-links between polymer chains through sulfur atoms, enhancing elasticity and mechanical strength. Sulfur donors act as controlled sulfur sources releasing active sulfur species gradually, enabling precise vulcanization with improved processing safety and reduced scorching. Free sulfur curing uses elemental sulfur directly, often resulting in faster cure rates but less control over cross-link density and potential for uneven vulcanization.
Overview of Sulfur Donor and Free Sulfur Systems
Sulfur donor curing systems utilize compounds that release sulfur gradually during vulcanization, providing controlled cross-linking and enhanced processing safety compared to free sulfur systems, which directly add elemental sulfur for faster curing but with higher risks of scorching. Sulfur donor systems improve the uniformity of cross-links and reduce reversion, leading to better mechanical properties and stability in rubber products. Free sulfur curing remains widely used for its simplicity and cost-effectiveness but requires careful handling to balance curing speed and product quality.
Chemical Mechanisms: Sulfur Donor vs Free Sulfur
Sulfur donor curing involves the use of sulfur-containing compounds that release sulfur gradually during vulcanization, leading to controlled cross-link formation and enhanced mechanical properties. Free sulfur curing relies on elemental sulfur directly interacting with polymer chains, creating polysulfidic bridges that influence elasticity and thermal stability. The chemical mechanism of sulfur donors offers improved process safety and reduced reversion compared to free sulfur, which can cause uneven cross-linking and premature aging of rubber.
Key Compounds in Sulfur Donor Curing
Sulfur donor curing relies on compounds such as sulfenamides, thiazoles, and dithiocarbamates to provide controlled sulfur release during vulcanization, enhancing the cross-linking process without the drawbacks of free sulfur. These key compounds facilitate better scorch safety and improved mechanical properties by promoting efficient sulfur transfer and reducing premature curing. Your rubber formulations benefit from more consistent cure rates and enhanced durability compared to traditional free sulfur curing systems.
Crosslinking Efficiency: Comparing Curing Agents
Sulfur donor curing agents offer enhanced crosslinking efficiency compared to free sulfur curing by providing a controlled release of sulfur during vulcanization, resulting in more uniform and stable crosslinks. Free sulfur often leads to inconsistent crosslink density, which can impact the mechanical properties and aging resistance of the rubber. Choosing sulfur donors optimizes Your rubber compounds for superior durability and performance in demanding applications.
Impact on Rubber Properties and Performance
Sulfur donor curing enhances rubber properties by providing a controlled cross-linking process that improves elasticity, tensile strength, and abrasion resistance compared to free sulfur curing. Free sulfur curing often results in uneven cross-link distribution, leading to inferior mechanical properties and reduced durability in rubber products. Your choice between these methods directly impacts the rubber's performance, with sulfur donor curing offering better heat aging stability and overall enhanced material consistency.
Processing Conditions and Curing Parameters
Sulfur donor curing uses specialized sulfur-containing compounds that release sulfur slowly during vulcanization, allowing for more controlled processing conditions and precise curing parameters, such as temperature typically ranging from 140-160degC and longer cure times. Free sulfur curing involves direct addition of elemental sulfur, leading to faster cross-linking reactions at higher temperatures, usually 160-180degC, with shorter cure times but less control over scorch safety and crosslink density. Your choice between sulfur donor and free sulfur curing affects the balance between processing flexibility, scorch safety, and final elastomer properties.
Advantages of Sulfur Donor Curing System
Sulfur donor curing systems offer precise control over vulcanization rates and crosslink density, resulting in improved mechanical properties and enhanced aging resistance compared to free sulfur curing. These systems minimize the formation of undesirable polysulfidic crosslinks, thereby reducing reversion and improving heat resistance in rubber products. The controlled sulfur release also enhances process safety and reduces volatility, making sulfur donor curing a preferred choice in high-performance elastomer applications.
Limitations and Challenges of Each Method
Sulfur donor curing often faces challenges such as slower cure rates and limited crosslink density compared to free sulfur curing, affecting overall vulcanizate properties. Free sulfur curing can lead to issues like reversion at high temperatures, resulting in weakened mechanical strength and reduced durability. Both methods require careful optimization to balance cure speed, crosslink structure, and thermal stability for specific rubber applications.
Industry Applications and Future Trends
Sulfur donor curing offers precise control over vulcanization in tire manufacturing and conveyor belts, reducing reversion and improving mechanical properties compared to free sulfur curing, which remains widely used in rubber gloves and seals for cost efficiency. Industry applications prioritize sulfur donor curing for high-performance products demanding durability and consistent cross-link density, while free sulfur curing suits general-purpose elastomers due to its simplicity. Your choice between these curing methods affects product quality and aligns with future trends emphasizing sustainability and enhanced material performance through advanced accelerator systems.
Sulfur donor vs Free sulfur curing Infographic
 libmatt.com
libmatt.com