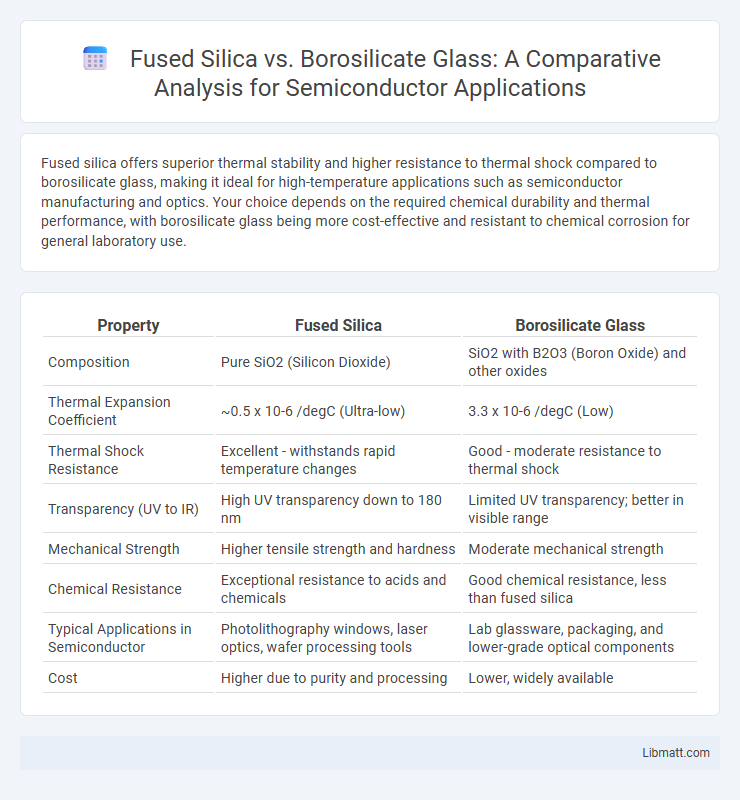
Fused silica offers superior thermal stability and higher resistance to thermal shock compared to borosilicate glass, making it ideal for high-temperature applications such as semiconductor manufacturing and optics. Your choice depends on the required chemical durability and thermal performance, with borosilicate glass being more cost-effective and resistant to chemical corrosion for general laboratory use.
Table of Comparison
| Property | Fused Silica | Borosilicate Glass |
|---|---|---|
| Composition | Pure SiO2 (Silicon Dioxide) | SiO2 with B2O3 (Boron Oxide) and other oxides |
| Thermal Expansion Coefficient | ~0.5 x 10-6 /degC (Ultra-low) | 3.3 x 10-6 /degC (Low) |
| Thermal Shock Resistance | Excellent - withstands rapid temperature changes | Good - moderate resistance to thermal shock |
| Transparency (UV to IR) | High UV transparency down to 180 nm | Limited UV transparency; better in visible range |
| Mechanical Strength | Higher tensile strength and hardness | Moderate mechanical strength |
| Chemical Resistance | Exceptional resistance to acids and chemicals | Good chemical resistance, less than fused silica |
| Typical Applications in Semiconductor | Photolithography windows, laser optics, wafer processing tools | Lab glassware, packaging, and lower-grade optical components |
| Cost | Higher due to purity and processing | Lower, widely available |
Introduction to Fused Silica and Borosilicate Glass
Fused silica is a high-purity silicon dioxide glass known for its exceptional thermal stability, low thermal expansion, and excellent optical clarity, making it ideal for applications requiring extreme heat resistance. Borosilicate glass contains silica and boron trioxide, offering enhanced chemical durability and resistance to thermal shock compared to traditional glass, widely used in laboratory equipment and cookware. Your choice between fused silica and borosilicate glass will depend on the specific demands of temperature tolerance, chemical stability, and optical performance.
Chemical Composition Differences
Fused silica is composed almost entirely of silicon dioxide (SiO2) with a very pure and uniform molecular structure, while borosilicate glass contains roughly 80% silica combined with about 13% boron trioxide (B2O3), along with small amounts of sodium oxide and aluminum oxide. The inclusion of boron trioxide in borosilicate glass enhances its thermal shock resistance and chemical durability compared to fused silica, which is highly resistant to high temperatures but more sensitive to rapid temperature changes. Your choice between these materials depends on the specific chemical and thermal demands of your application.
Thermal Properties Comparison
Fused silica exhibits an extremely low coefficient of thermal expansion (~0.5 x 10^-6 /degC), enabling superior thermal shock resistance and stability at high temperatures up to 1200degC. Borosilicate glass has a higher thermal expansion (~3.3 x 10^-6 /degC) but offers good resistance to thermal stress and can operate safely up to about 500degC. If your application demands minimal dimensional change under rapid temperature fluctuations, fused silica provides optimal performance due to its outstanding thermal properties.
Optical Clarity and Transmission
Fused silica exhibits superior optical clarity and higher ultraviolet (UV) transmission compared to borosilicate glass, making it ideal for precision optics and UV applications. Borosilicate glass offers good clarity but has a lower UV transmission cutoff around 300 nm, whereas fused silica transmits light down to approximately 180 nm. Your choice between these materials should consider the required wavelength range and clarity for optimal performance in optical systems.
Mechanical Strength and Durability
Fused silica exhibits superior mechanical strength and durability compared to borosilicate glass due to its high purity and exceptional thermal shock resistance. Its low coefficient of thermal expansion minimizes stress fractures, making it ideal for high-stress environments and precision applications. Borosilicate glass provides good mechanical strength and chemical resistance but tends to be less durable under extreme temperature changes and mechanical impact.
Resistance to Thermal Shock
Fused silica exhibits superior resistance to thermal shock due to its extremely low coefficient of thermal expansion, typically around 0.5 x 10^-6 /degC, allowing it to withstand rapid temperature changes without cracking. Borosilicate glass has a higher coefficient of thermal expansion, approximately 3.3 x 10^-6 /degC, which makes it less resistant to sudden temperature fluctuations compared to fused silica. This difference makes fused silica the preferred choice for high-precision applications requiring exceptional thermal stability.
Common Applications in Industry
Fused silica is widely used in industries requiring high thermal shock resistance and purity, such as semiconductor manufacturing, optical fiber production, and high-precision laboratory equipment. Borosilicate glass finds common applications in chemical laboratories, cookware, and lighting due to its excellent chemical durability and moderate thermal resistance. Understanding the specific requirements of your industrial application helps determine whether the superior thermal stability of fused silica or the versatile chemical resistance of borosilicate glass is more suitable.
Cost and Availability
Fused silica is generally more expensive than borosilicate glass due to its high purity and superior thermal properties, making it less common in everyday applications. Borosilicate glass is widely available and cost-effective, favored for general laboratory and industrial use because it balances durability and affordability. The higher production costs and specialized manufacturing of fused silica limit its availability compared to the more commonly produced borosilicate glass.
Environmental and Safety Considerations
Fused silica offers superior thermal shock resistance and lower thermal expansion, reducing the risk of breakage in extreme temperature conditions, which enhances your safety during use. Borosilicate glass, while less resistant to rapid temperature changes, generally contains fewer impurities and offers better chemical durability under normal environmental conditions. Choosing between these materials depends on your specific safety needs and the environmental stresses your application will face.
How to Choose: Fused Silica or Borosilicate Glass?
Choosing between fused silica and borosilicate glass depends on your application's temperature resistance, chemical durability, and optical clarity requirements. Fused silica offers superior thermal stability up to 1,200degC, excellent UV transparency, and low thermal expansion, ideal for high-precision optics and semiconductor processes. Borosilicate glass provides good thermal shock resistance and chemical durability at a lower cost, making it suitable for general laboratory glassware and everyday cookware; consider your operating environment and budget to determine the best option for your needs.
Fused Silica vs Borosilicate Glass Infographic
 libmatt.com
libmatt.com